Atrophy is a simple and opposing process to hypertrophy. Just as exercise can drive an adaptive increase in muscle mass, lack of exercise can cause a reductive adaptation or loss of muscle mass. As one would expect, the entire muscle may shrink in size such that it is easily and visually detectable, but atrophy occurs at every level of muscular organization, beginning with the molecular.
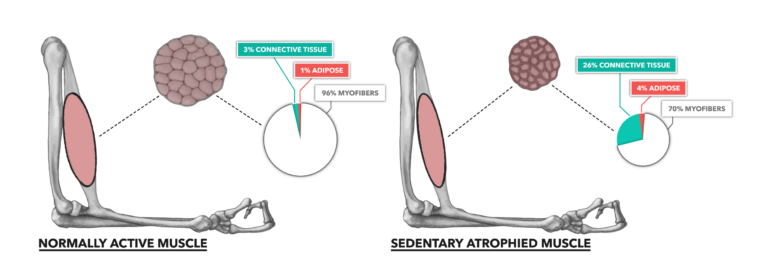
Figure 1: Normally active bicep compared to a sedentary, atrophied bicep. Muscle atrophy is the loss of muscle mass over time. The majority of dimensional decay comes in the form of lost myofibrillar content.
Atrophy is mostly driven by inactivity. When there is no stimulus to move the body, there is no biological impetus to maintain or increase muscle mass. This sets the stage for a loss of contractile proteins, regulatory proteins, and metabolic resources within the sedentary cell. The most observable change noted in atrophy is the large scale loss of myofibrillar content. This is strongly associated with reduced contractile function.
Atrophy can become more severe with continued inactivity and age, and it can result in the loss of entire muscle cells. This reduction in cell number within a muscle is called sarcopenia. While myofibrillar atrophy is largely a recoverable condition, once there is an onset of sarcopenia, redevelopment of muscle cells does not occur. This means permanent losses in movement function and metabolic capacity.
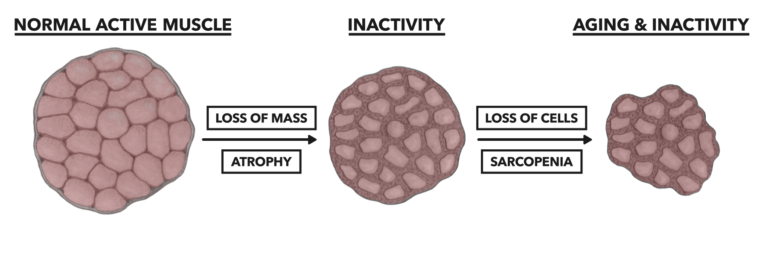
Figure 2: Sarcopenia, the disappearance of entire cells from the muscle, is most associated with aging populations and potentially affects up to 33% of older populations.
One major issue regarding sarcopenia is that it can only be estimated, indirectly, in clinical environments. The most common measures are the same used to measure hypertrophy and atrophy — i.e., dimensional measures such as girth measurement, body composition measurement, and MRI/CT/DEXA imaging. Blood chemistry has been used as a proxy measure and is somewhat common for evaluating muscle strength in a clinical context. Simple hand grip dynamometers or, more uncommonly, isokinetic testing devices are used to acquire functional data to indirectly diagnose architectural change. Sarcopenia is diagnosed without actually counting muscle cell number; rather, atrophy and functional decay (weakness) are used (1).
REFERENCES
- Shaw SC, Dennison EM, Cooper C. Epidemiology of sarcopenia: Determinants throughout the lifecourse. Calcified Tissue International 101.3(2017): 229–247.
- Kern H, Hofer C, Modlin M, Mayr W, Vindigni V, Zampieri S, Boncompagni S, Protasi F, Carraro U. Stable muscle atrophy in long-term paraplegics with complete upper motor neuron lesion from 3- to 20-year SCI. Spinal Cord 46(2008): 293–304.
Comments on Muscle Basics, Part 4: Atrophy & Sarcopenia
I disagree with the use of the word sarcopenia and the implication in the article. Our quasi researchers are going to have to update their outdated beliefs. Determining when sarcopenia sets in has never been established.
Hi, small suggestion for multi-part articles: add a clear list of links to the other parts (part 1, 2, 3).
Just noticed that on part 3 there's a section "additional reading" doing just that… perfect! ;-)
Muscle Basics, Part 4: Atrophy & Sarcopenia
3