In this influential 2009 paper, Matthew Vander Heiden, Lewis Cantley, and Craig Thompson provide an alternative explanation for the Warburg effect. The authors argue that cancer cells rely heavily on aerobic glycolysis, not for reasons related to energy requirements, but rather to create the substrates for rapid cell proliferation.
As background: A glucose molecule can be broken down through three processes relevant to this discussion. Oxidative phosphorylation is the primary process normal cells use in the presence of oxygen and delivers 36 molecules of ATP for each molecule of glucose consumed. When a normal cell lacks oxygen, it is forced to use anaerobic glycolysis, which breaks glucose down into lactate and only generates two molecules of ATP for each molecule of glucose. Cancer cells instead use aerobic glycolysis, breaking glucose down to lactate even when oxygen is present and delivering four molecules of ATP for each molecule of glucose consumed.
Vander Heiden et al. first note an analogy between cancer cells and unicellular organisms. While unicellular organisms are in an environment with abundant nutrients, they gear metabolism toward processes that generate biomass, ethanol, and organic acids to encourage proliferation; conversely, when energy is scarce, they seek to maximize the energy extracted from available nutrients. In multicellular organisms, uncontrolled proliferation generally is suppressed, so cells maximize energy extraction rather than biomass production. Cancer cells, on the other hand, take in and use nutrients like a unicellular organism in a proliferative state.
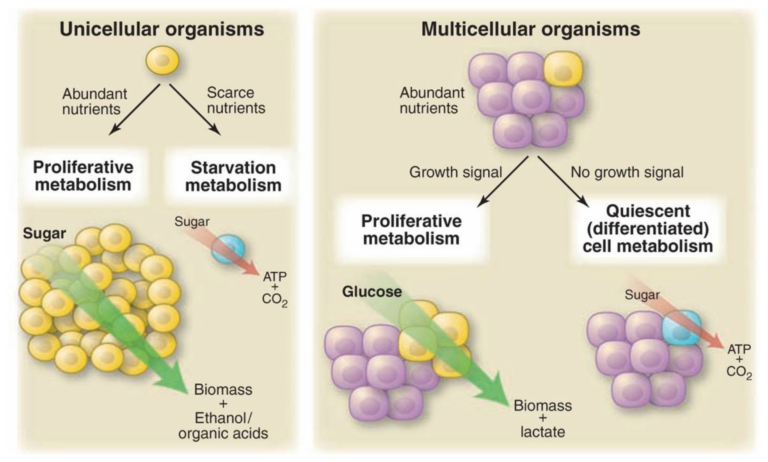
Figure 1, Microbes and cells from multicellular organisms have similar metabolic phenotypes under similar environmental conditions. Unicellular organisms undergoing exponential growth often grow by fermentation of glucose into a small organic molecule such as ethanol. These organisms, and proliferating cells in a multicellular organism, both metabolize glucose primarily through glycolysis, excreting large amounts of carbon in the form of ethanol, lactate, or another organic acid such as acetate or butyrate. Unicellular organisms starved of nutrients rely primarily on oxidative metabolism, as do cells in a multicellular organism that are not stimulated to proliferate. This evolutionary conservation suggests that there is an advantage to oxidative metabolism during nutrient limitation and nonoxidative metabolism during cell proliferation. From Understanding the Warburg Effect,The Metabolic Requirements of Cell Proliferation.
In 1924, Otto Warburg noted that cancer cells, unlike normal tissues, ferment glucose into lactate even when there is sufficient oxygen to support oxidative phosphorylation (i.e., they perform aerobic glycolysis). In other words, cancer cells metabolize glucose, using pathways that generate less energy (ATP) per glucose molecule consumed despite having the resources necessary to use a more efficient process. While the observation is widely recognized, there is no recognized explanation for why cancer cells behave this way.
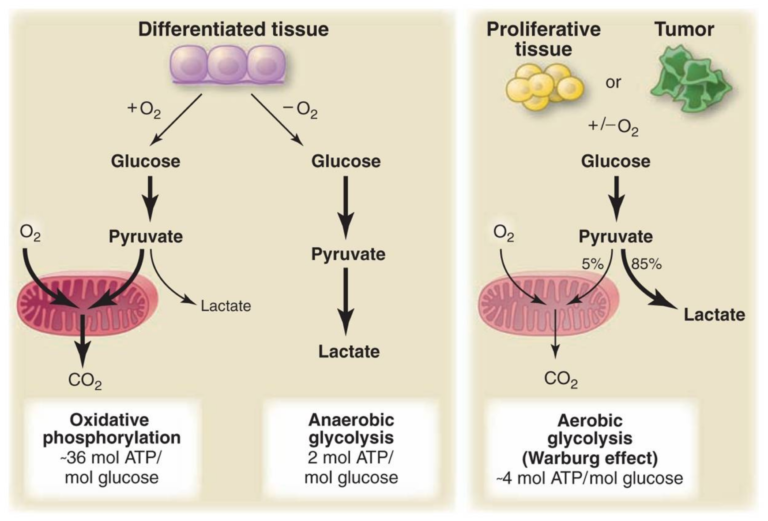
Figure 2, Schematic representation of the differences between oxidative phosphorylation, anaerobic glycolysis, and aerobic glycolysis (Warburg effect). In the presence of oxygen, nonproliferating (differentiated) tissues first metabolize glucose to pyruvate via glycolysis and then completely oxidize most of that pyruvate in the mitochondria to CO2 during the process of oxidative phosphorylation. Because oxygen is required as the final electron acceptor to completely oxidize the glucose, oxygen is essential for this process. When oxygen is limiting, cells can redirect the pyruvate generated by glycolysis away from mitochondrial oxidative phosphorylation by generating lactate (anaerobic glycolysis). This generation of lactate during anaerobic glycolysis allows glycolysis to continue (by cycling NADH back to NAD+), but results in minimal ATP production when compared with oxidative phosphorylation. Warburg observed that cancer cells tend to convert most glucose to lactate regardless of whether oxygen is present (aerobic glycolysis). This property is shared by normal proliferative tissues. Mitochondria remain functional and some oxidative phosphorylation continues in both cancer cells and normal proliferating cells. Nevertheless, aerobic glycolysis is less efficient than oxidative phosphorylation for generating ATP. In proliferating cells, ~10% of the glucose is diverted into biosynthetic pathways upstream of pyruvate production. From Understanding the Warburg Effect,The Metabolic Requirements of Cell Proliferation.
The rapid growth of cancer cells (and closer mechanistic studies) demonstrates that their use of aerobic glycolysis does not prevent them from generating sufficient energy to survive and reproduce. Instead, the authors argue, the more pressing constraint on proliferation may be the production of the nucleotides, amino acids, and lipids necessary to replicate the entire contents of the cell before mitosis. While production of these raw constituents of growth, such as palmitate and amino acids, require some ATP, their requirements for carbon and other substrates are greater relative to what glucose can provide.
As an example of these requirements: “to make a 16-carbon fatty acyl chain, a single glucose molecule can provide five times the ATP required, whereas 7 glucose molecules are needed to generate the NADPH required.” In other words, if the majority of glucose is committed to ATP production, the cell will have insufficient substrate to rapidly replicate its contents and proliferate. This is true for a variety of molecules, as glucose and glutamine “supply most of the carbon, nitrogen, free energy and reducing equivalents necessary to support cell growth and division.”
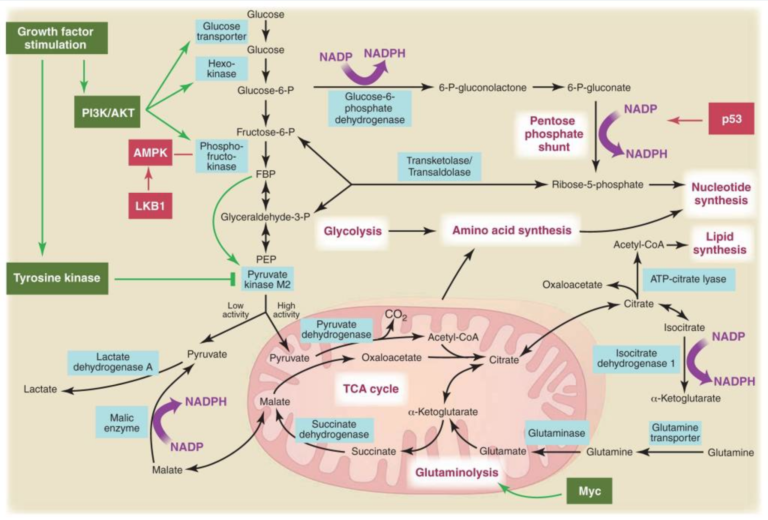
Figure 3, Metabolic pathways active in proliferating cells are directly controlled by signaling pathways involving known oncogenes and tumor suppressor genes. This schematic shows our current understanding of how glycolysis, oxidative phosphorylation, the pentose phosphate pathway, and glutamine metabolism are interconnected in proliferating cells. This metabolic wiring allows for both NADPH production and acetyl-CoA flux to the cytosol for lipid synthesis. Key steps in these metabolic pathways can be influenced by signaling pathways known to be important for cell proliferation. Activation of growth factor receptors leads to both tyrosine kinase signaling and PI3K activation. Via AKT, PI3K activation stimulates glucose uptake and flux through the early part of glycolysis. Tyrosine kinase signaling negatively regulates flux through the late steps of glycolysis, making glycolytic intermediates available for macromolecular synthesis as well as supporting NADPH production. Myc drives glutamine metabolism, which also supports NADPH production. LKB1/AMPK signaling and p53 decrease metabolic flux through glycolysis in response to cell stress. Decreased glycolytic flux in response to LKB/AMPK or p53 may be an adaptive response to shut off proliferative metabolism during periods of low energy availability or oxidative stress. Tumor suppressors are shown in red, and oncogenes are in green. Key metabolic pathways are labeled in purple with white boxes, and the enzymes controlling critical steps in these pathways are shown in blue. Some of these enzymes are candidates as novel therapeutic targets in cancer. Malic enzyme refers to NADP+-specific malate dehydrogenase [systematic name (S)-malate:NADP+ oxidoreductase (oxaloacetate-decarboxylating)]. From Understanding the Warburg Effect,The Metabolic Requirements of Cell Proliferation.