In this 2017 review, Matthew Vander Heiden (with Ralph DeBerardinis) expands on his 2009 interpretation of the role metabolic defects play in the growth and progression of cancer cells and explains how these defects and mechanisms may be targeted to slow cancer’s advance.
While the Warburg effect (i.e., the underuse of respiration) is the most well-documented distortion in cancer metabolism, recent research has found additional distortions in metabolism related to acetate, branched chain amino acids, serine, and glycine. These distortions demonstrate that cancers can use a variety of fuels to support growth.
The authors divide the many mutations present in cancer into three forms. Transforming activities (e.g., mutations in tumor suppressor genes and some metabolic mutations) directly contribute to the development of cancer. Enabling activities (many of which accelerate nutrient uptake or processing) support tumor cell growth but are not involved in tumorigenesis. Neutral activities may be present in cancers but are not responsible for their growth or proliferation. Consequently, only mutations in the former two categories are therapeutic targets.
g
ATP generation does not seem to be a rate-limiting step in tumor growth, as is evidenced both by aspects of cell behavior (e.g., use of aerobic glycolysis) and the fact that increasing ATP consumption promotes proliferation. Similarly, NADPH availability does not limit tumor growth. TCA cycle products (e.g., glutamine and other amino acids) and nucleotide synthesis, however, are rate-limiting steps.
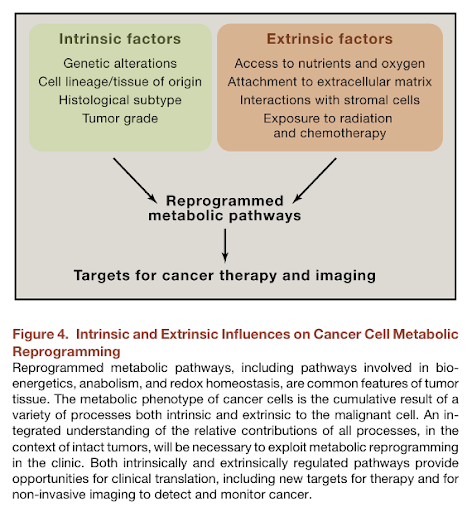
Understanding the Intersections Between Metabolism and Cancer Biology