This 2016 paper reviewed the effects and potential clinical implications of the ketogenic diet.
The authors describe four distinct variations of the ketogenic diet (1):
- The “traditional” ketogenic diet (long-chain triglyceride) in which as much as 80% of calories come from fat, and both total calories and protein are restricted to maximize blood glucose suppression and ketone elevation
- The MCT diet, which combines significant carbohydrate restriction with MCT oil supplementation (MCT oil converts rapidly into ketones, so supplementary MCT oil supports nutritional ketosis with less intensive carbohydrate restriction)
- The modified Atkins diet, which is similar to the “traditional” ketogenic diet in that it restricts carbohydrate, but it does so less severely and without any restriction on total calorie intake
- The LGIT (low-glycemic-index treatment), which does not restrict total carbohydrate intake but instead restricts all carbs to only those with a glycemic index of less than 50 to reduce blood glucose spikes associated with high-glycemic-index carb intake
All these diets, as well as fasting, achieve a similar biological state — a significant reduction in blood glucose levels and a parallel increase in blood ketone levels (conversely, any diet that does not suppress glucose levels and elevate ketone levels is not a ketogenic diet). More restrictive ketogenic diets, however, may lead to greater elevations in blood ketone levels. The figure below shows how ketones are produced.
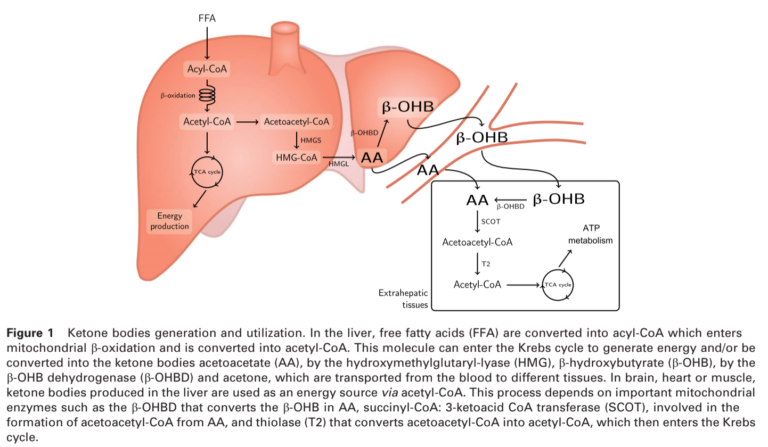
Two effects of a ketogenic diet — blood glucose suppression and blood ketone elevation — may prove beneficial to cancer treatment. Cancer cells, as discussed elsewhere on CrossFit.com, are dependent on high levels of glucose consumption, both for energy and to support cellular maintenance, growth, and proliferation (2). The ketogenic diet dramatically suppresses glucose availability and starves cancer cells while allowing healthy cells to switch to ketones for fuel. While a ketogenic diet induces weight loss, which is often a concern in cancer patients, it has been shown to preserve lean body mass and thus to prevent cachexia (3).
The ketogenic diet may also help treat a variety of neurological diseases (4). It has been used to successfully treat epilepsy since the 1920s, long before the mechanism of action was established; it is now understood that lower levels of glucose and higher levels of blood ketones normalize mitochondrial function and thus reduce the neuronal hyperexcitability that leads to seizures. The ketogenic diet has also been hypothesized as a treatment for Alzheimer’s, Parkinson’s, ALS, and other conditions — all of which have been linked to deficiencies in brain metabolism that may be resolved by a ketogenic state, though this research remains preliminary (5).
Finally, the ketogenic diet has specific benefits relevant to a variety of rare genetic mitochondrial defects (6). The ketogenic state supports improved mitochondrial function and biosynthesis while also reducing the body’s reliance on glucose metabolism. It thereby minimizes the impact of defects in glucose metabolism (7).
With the exception of epilepsy (where the utility of ketogenic diets is well established), the evidence supporting the clinical use of ketogenic diets is limited. However, these conditions lack an effective standard of care, and in each case, preliminary (i.e., in vitro or animal) data suggests ketogenic diets may have clinically significant benefits. As such, future research to understand the magnitude, consistency, and breadth of the effects of a ketogenic diet in cancer, neurological disease, and other specific conditions remains warranted.
Notes
- Neuroactive peptides as putative mediators of antiepileptic ketogenic diets
- The metabolism of tumors in the body; Understanding the Warburg effect: the metabolic requirements of cell proliferation; Is there a role for carbohydrate restriction in the treatment and prevention of cancer?; Overexpression of Glut-1 and increased glucose metabolism in tumor are associated with a poor prognosis in patients with oral squamous cell carcinoma; Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma
- Cancer cachexia: Influence of systemic ketosis on substrate levels of nitrogen metabolism; Implications of ketogenic diets on weight gain, motor activity and cicatrization in Wistar rats
- The ketogenic diet as a treatment paradigm for diverse neurological disorders; The ketogenic diet: metabolic influences on brain excitability and epilepsy; The ketogenic diet in children, adolescents and young adults with refractory epilepsy; The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial; Do patients with absence epilepsy respond to ketogenic diets? A prospective study of the modified Atkins diet for intractable epilepsy in adults; Ketone bodies mediate antiseizure effects through mitochondrial permeability transition
- A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease; A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease; Treatment of Parkinson’s disease with a diet-induced hyperketonemia: A feasibility study; Dietary fatty acids and the risk of Parkinson disease: the Rotterdman study; A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis; Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis
- Mitochondrial energetics and therapeutics; Mitochondrial disease and epilepsy
- Mitochondrial myopathies: Developments in treatment; Ketogenic diet slows down mitochondrial myopathy progression in mice