Gerald Reaven sets out to discover what insulin resistance syndrome (IRS) is
In the previous column (1), I explained that Gerald Reaven began his research of insulin resistance syndrome (IRS) because he wanted to understand what Harry Himsworth meant when he proposed that the metabolic defect in the commoner form of diabetes is an insensitivity of the patient’s tissues to the actions of insulin (2). In the process, Reaven discovered the work of Margaret Albrink and her colleagues (3), which showed that persons with coronary heart disease (CHD), including those with Type 2 diabetes mellitus (T2DM), are rather more likely to have elevated blood triglyceride than blood cholesterol concentrations. This finding ran contrary to the idea then gaining global credence: that elevated blood cholesterol concentrations are the singular cause of CHD. At the same time, Peter Kuo in Philadelphia was showing that high-carbohydrate diets, especially those containing sucrose or fructose, caused an increase in blood triglyceride concentrations (hypertriglyceridemia), particularly in those who are carbohydrate-sensitive (4). Thus, Kuo coined the term “carbohydrate-sensitive hypertriglyceridemia” (CSHT).
This led Reaven to ask the question: Why do carbohydrate-sensitive persons with insulin-resistant T2DM have elevated blood triglyceride concentrations?
Why blood triglyceride concentrations are elevated in persons with T2DM
Reaven began his research journey with the popular understanding of the day that T2DM is a key driver of arterial disease, especially of the coronary arteries, thus leading to coronary heart disease (CHD). This was the concept that, I suspect, was then being taught at most of the world’s medical schools, but the next 60 years would witness a radical change. Future generations instead would be taught Ancel Keys’ false lipid hypothesis, which holds that an elevated blood cholesterol concentration is the only important blood (bio)chemical driver of CHD.
So when those with T2DM developed CHD, the explanation offered by the experts was as simple then as it is today: The main cause is elevated blood cholesterol concentrations. The evidence Albrink and her colleagues presented to show blood triglyceride and not blood cholesterol concentrations were more likely to be raised in persons with T2DM and CHD was simply ignored — and ultimately suppressed and then forgotten (as it is today).
This knowledge was forgotten even though other researchers (5-9) had come to exactly the same conclusion by the time Reaven and colleagues completed their studies of the topic in 1994.
Reaven’s first important study, published in 1963 (10), evaluated carbohydrate metabolism in 41 patients with documented myocardial infarction (MI). He found that carbohydrate metabolism was impaired in MI patients compared to controls — that is, MI patients were more insulin resistant. He also observed that MI patients had higher serum triglyceride and cholesterol levels. He concluded, “The apparent presence of minimal abnormalities of carbohydrate metabolism in a significant number of patients with arteriosclerotic heart disease warrants further consideration as a possible factor in the development of atherosclerosis” (10, p. 1013, my emphasis). He noted that four other studies had already recognized this relationship:
Although the number of patients from the infarction group with a positive glucose tolerance test seems quite high (41%), the existence of abnormal carbohydrate metabolism in patients with atherosclerosis has been observed by Sohrade, Boehle and Bieglee (11), Waddell and Field (12), Sowton (13) and Wahlberg (14). Although all these studies differed in the nature of the patients selected, composition of the control group, glucose tolerance test used, time tested after infarction, and other factors, there is considerable degree of similarity between the results. (10, p. 1019)
Patients with higher blood triglyceride concentrations were more insulin resistant than controls, but Reaven was unable to demonstrate a clear link between higher levels of insulin resistance and hypertriglyceridemia. Thus, the cause of hypertriglyceridemia in these MI patients was not established.
However, others were already showing that persons with hypertriglyceridemia were more likely to be resistant to the glucose-lowering effects of injected insulin (15). That is, persons with hypertriglyceridemia required the injection of more insulin to lower their blood glucose concentrations.
Next, Reaven developed the methods to measure rates of liver triglyceride production (16). These rates were then measured in a range of persons with different blood triglyceride concentrations. In a second study (17), a group of 33 clinic patients were fed a high-carbohydrate diet (85%) for three weeks, at the end of which, 29 subjects had markedly elevated blood triglyceride concentrations (>300 mg/dL; >3.4 mmol/L).
These studies showed a linear relationship between the rates of liver triglyceride production and the log of the blood (plasma) triglyceride concentrations (Figure 1; left panel). They showed a similar linear relationship between plasma triglyceride concentrations and blood insulin concentrations.
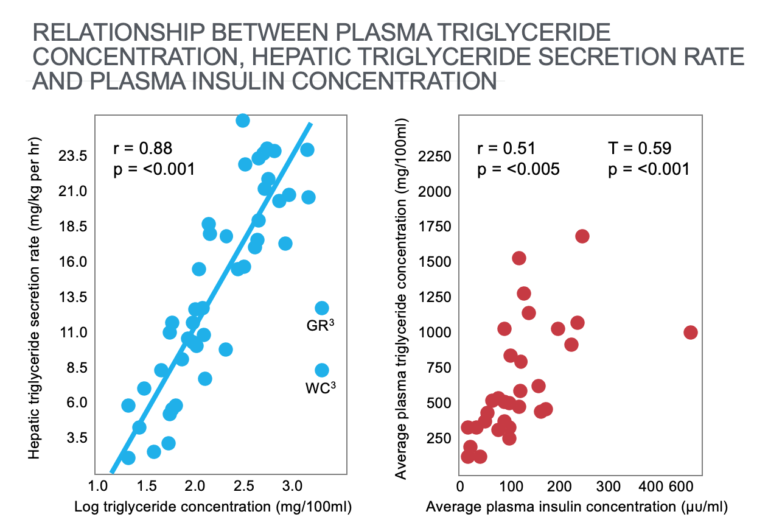
Figure 1: The left panel shows a significant linear relationship between the rates of hepatic (liver) triglyceride production and the log of plasma (blood) triglyceride concentrations. The right panel shows a significant relationship between plasma triglyceride and plasma insulin concentrations. Reproduced from reference 17.
Note that a healthy blood triglyceride concentration is below 88 mg/dL (1 mmol/L). Thus, the overwhelming majority of subjects in this study were markedly hypertriglyceridemic.
Thus, the primary cause of hypertriglyceridemia in these studies appeared to be “carbohydrate-induced increases in hepatic triglyceride secretion rates” (17, p. 1765), which was in turn “highly correlated with the plasma insulin response produced by that diet” (p. 1766). Interestingly there was no relationship between degree of obesity and the extent of this carbohydrate-induced hypertriglyceridemia.
A subsequent study seven years later (18) confirmed all these findings. It added the additional finding that the stable blood triglyceride concentrations in persons all eating the same diet was predicted by differences in their levels of insulin resistance, which determined their insulin responses to carbohydrate ingestion. Once again, obesity did not predict any of these relationships.
One of the forgotten gems from this 1974 paper is that the metabolic responses of subjects were measured when they ingested a diet that “attempts to approximate the constituents of the average American diet” of that time. The diet comprised 43% carbohydrate and 42% fat (18, p. 552). In today’s language, that would be described (incorrectly) as a low-carbohydrate, high-fat diet.
During this period, Reaven’s group also evaluated the effects on blood triglyceride concentrations of eating high- and low-carbohydrate diets (85% and 17%, respectively) for between two and 10 weeks (19). As shown in Figure 2, blood triglyceride concentrations doubled on the high-carbohydrate diet, whereas blood cholesterol and phospholipid values were less affected. Note that even on the 0%-carbohydrate diet, the majority of patients had quite markedly elevated blood triglyceride concentrations, with only two subjects recording desirable values of <88 mg/dL (<1 mmol/L).
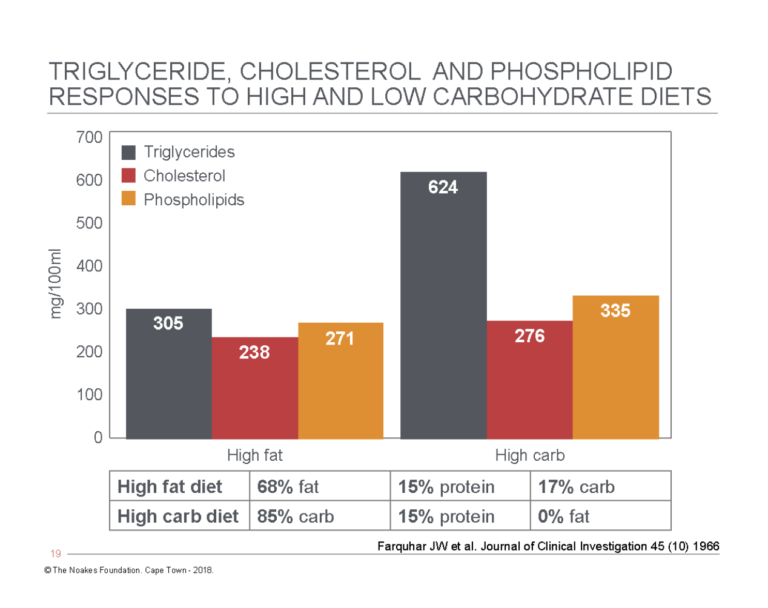
Figure 2: Comparison of blood triglyceride, cholesterol, and phospholipid concentrations in the same subjects when they ate either a high-fat, low-carbohydrate diet (left panels) or a high-carbohydrate, low-fat diet (right panels) for 10 weeks. Note that blood triglyceride concentrations more than doubled on the high-carbohydrate, low-fat diet, whereas the increases in blood cholesterol and phospholipid concentrations were more modest. Redrawn from data in Table 2, reference 19.
The study found that all measures of glucose and insulin responses to carbohydrate ingestion correlated “very well with triglyceride response” (p. 1652-3). Thus, the authors explained, “The more abnormal the glucose tolerance test and the higher the accompanying rises in plasma insulin-like activity and immune-reactive insulin, the greater the subsequent triglyceride rise on ingestion of a high carbohydrate diet” (p.1654). Once again, the indication was that the hypertriglyceridemic response was related to the individual’s degree of insulin resistance.
The authors concluded, “The observed elevations of plasma glucose, insulin-like activity, and immunoreactive insulin correlated well with the magnitude of the triglyceride response that resulted from the subsequent ingestion of the higher carbohydrate diet.” They therefore ultimately suggested, “Hyperinsulinemia, in the presence of normal to moderately elevated levels of plasma glucose, may be an important cause of the enhanced hepatic triglyceride production that underlies endogenous hypertriglyceridemia” (p. 1655).
As I described in the previous column (1), at precisely the same time, Kuo in Philadelphia was showing that “a high incidence of carbohydrate-sensitive hyperglyceridemia could be demonstrated in persons with atherosclerosis” (4, p. 92).
So, already by 1967, Reaven’s research group had established the following:
- When tested appropriately, a significant proportion of persons with coronary artery disease (atherosclerosis) have abnormalities in carbohydrate metabolism.
- The key abnormality appears to be elevated blood triglyceride concentrations due in part to higher levels of insulin resistance.
- Higher blood triglyceride concentrations were due to higher rates of hepatic (liver) triglyceride production.
- Higher rates of liver triglyceride production were due to higher blood insulin concentrations.
- Blood triglyceride concentrations increased on a very high-carb, low-fat diet (85% and 0%, respectively).
- Blood triglyceride concentrations were lower on a lower-carbohydrate, higher-fat diet (17% and 68%, respectively).
- The response of blood triglyceride concentrations appeared to be explained by individual differences in carbohydrate tolerance (insulin resistance).
Most of the future work performed by Reaven and his group would be focused on the description of this condition (insulin resistance/carbohydrate intolerance) and its role in causing the majority of our modern chronic “lifestyle” diseases. We will conclude this column with an analysis of his groups’ studies of the influence of diet, in particular carbohydrate content, on hypertriglyceridemia and other markers of impaired carbohydrate metabolism.
The role of low-carbohydrate diets in the management of T2DM
Over a seven-year period between 1987 and 1994, Reaven and his colleagues published three papers (20-23) that evaluated the effects of diets with different carbohydrate contents on blood parameters, specifically in persons with T2DM.
Without exception, these studies showed that removing carbohydrates from the diet uniformly improved measures of metabolic health in those with T2DM. Conversely, increasing the carbohydrate content of the diets produced uniformly detrimental effects.
In the first study, nine patients with T2DM followed diets with higher (60%) or lower (40%) carbohydrate contents for 15 days each (20). The authors made the point that the 40%-carbohydrate diet was, at the time, reflective of what Americans were eating, whereas the 60%-carbohydrate diet was the diet promoted by the American Diabetes Association (ADA) for persons with T2DM in order to produce a “fall in plasma low-density lipoprotein (LDL) cholesterol concentration and thus a reduction in the risk of coronary heart disease” (p. 214).
But the authors noted there was no evidence that a 60%-carb diet was advisable. Instead, they cited a range of studies showing that in person with T2DM, a higher-carbohydrate diet was known to cause “hyperglycemia, hyperinsulinemia, hypertriglyceridemia, and reduced plasma HDL cholesterol concentrations (all of which) have been identified as factors predisposing to the risk of coronary artery disease” (p. 214).
They warned: “Thus, there appears to be evidence that the dietary recommendations of the ADA may actually increase the risk (of) coronary artery disease in patients with T2DM” (p. 214).
The important point is that Reaven was saying a diet that raised blood triglyceride concentrations would increase the risk of coronary artery disease, even if it lowered the blood cholesterol concentrations.
The key findings were that eating the higher-carbohydrate diet produced the precise outcomes the authors believed to be detrimental, specifically “an increase in fasting and postprandial triglyceride concentrations, a deterioration in glycemic control … and a fall in plasma HDL-cholesterol concentrations” (p. 216). Worse, “the decrease in dietary fat intake associated with the 60 percent carbohydrate diet did not result in lower LDL cholesterol concentrations” (p. 216).
The authors concluded:
The 60 percent carbohydrate diet did not have the beneficial effect on LDL metabolism that was predicted and aggravated the defects in glucose, lipid and lipoprotein metabolism that are characteristic of NIDDM (non-insulin-dependent diabetes mellitus or T2DM). Furthermore, it should be emphasized that these untoward changes were noted despite the fact that the 60 percent carbohydrate diet contained almost twice as much (dietary) fiber. (p. 216-217)
Further, because of their interest in the triglyceride-raising effects of carbohydrates, they continued to focus their attention on what they then considered to be the key question: What are the likely long-term health consequences of this carbohydrate-induced deterioration in glycemic control, the carbohydrate-induced hypertriglyceridemia, and the carbohydrate-induced reduction in blood HDL-cholesterol concentrations?
They began by drawing attention to three studies (5-7) showing hypertriglyceridemia is a significant risk factor for CHD in patients with T2DM and noted: “It seems inappropriate to dismiss the current findings on the presumption that elevated triglyceride concentrations in patients with NIDDM are of no clinical significance” (p. 218). In fact, all three studies showed plasma triglyceride concentrations were more important CHD risk factors than cholesterol (7, p. 351).
Next, they quoted a study (26) linking the degree of hyperglycemia and damage to small arteries (which would include the arteries supplying the retina and the kidneys) and posed this question: “Even if the significance of this relationship is debated, could it be argued that the best diet for patients with NIDDM is one that accentuates the magnitude of their hyperglycemia?” (p. 218)
Finally, they noted that even a small (carbohydrate-induced) reduction in blood HDL-cholesterol concentrations had been associated with “significantly increased risk of coronary artery disease (27). Consequently, it seems to us that the burden of proof is on those who would argue that the effects of a 60 percent carbohydrate diet on HDL cholesterol is of no clinical significance” (p. 218).
They finalized their conclusions with a challenge to the ADA:
These results document that low-fat (20%), high-carbohydrate (60%) diets, containing moderate amounts of sucrose, similar in composition to the recommendations of the American Diabetes Association, have deleterious metabolic effects when consumed by patients with NIDDM for 15 days. Until it can be shown that these untoward effects are evanescent, and that long-term ingestion of similar diets will result in beneficial metabolic changes, it seems prudent to avoid the use of low-fat, high-carbohydrate diets containing moderate amounts of sucrose in patients with NIDDM. (20, p. 213)
The next study from this research group repeated a study identical to the previous study but increased the dietary intervention periods from 15 days to six weeks (21). The findings were essentially identical and showed that persons with NIDDM do not “adapt” to the negative metabolic consequences of eating a low-fat, high-carbohydrate diet.
Thus, the authors again concluded:
The results of this study indicate that high-carbohydrate diets lead to several changes in carbohydrate and lipid metabolism in patients with NIDDM that could lead to an increased risk of coronary artery disease, and these effects persist for >6 weeks. Given these results, it seems reasonable to suggest that the routine recommendation of low-fat high-carbohydrate diets for patients with NIDDM be reconsidered. (21, p. 94)
In their final study, published in 1994, the authors investigated the effects of the metabolic parameters of two different diets — the first high in carbohydrate (55%) and moderate in fat (30%); the second lower in carbohydrate (40%) and higher in fat (45%), with the added fat coming from monounsaturated fatty acids (22). Once again, the control diet was designed to match the ADA guidelines of the day.
And once again, the findings were identical to those of the other studies:
In NIDDM patients, high-carbohydrate diets compared with high-monounsaturated-fat diets caused persistent deterioration of glycemic control and accentuation of hyperinsulinemia, as well as increased plasma triglyceride and very-low-density lipoprotein cholesterol levels, which may not be desirable. (22, p. 1421)
The authors again warned:
We conclude that high-carbohydrate diets in NIDDM patients may cause persistent increase in plasma triglyceride and VLDL cholesterol levels, hyperinsulinemia, and deterioration in glycemic control; all of these metabolic changes may be deleterious and have the potential to accelerate atherosclerosis as well as microangiopathy. … Diets with higher proportions of cis-monounsaturated fats may be advantageous in reducing the long-term complications, particularly heart disease, in NIDDM patients. (p. 1427)
Reaven fails to ask the crucial question
So, the key point is that by 1994, Reaven and his group were on the brink of discovering the optimum treatment for the very condition — the insulin resistance syndrome (IRS), including T2DM and what Reaven would call “Syndrome X” — that his remarkable research group would discover and define over the next 20 years.
The treatment they would have “discovered” was a very low-carbohydrate (5-10%) diet.
But they failed to ask the key question: If higher-carbohydrate diets (60%) induce an abnormal metabolic profile in those with IRS, whereas lower-carbohydrate diets (40%) have a less damaging effect, what would happen if we lowered the carbohydrate content even lower. Say to below 20%? Or perhaps even below 10%? Or as low as 5%?
The result was that between 1994 and when he passed away in 2018, Reaven would never promote a genuinely low-carbohydrate diet for the management of IRS, T2DM, or Syndrome X.
Instead he would, in my opinion and as I describe in the next column, drop the dietary “ball.” On the edge of a stunning medical victory and with perhaps a real shot at the Nobel Prize, he would snatch defeat right out of the jaws of victory.
By failing to ask the key question, he delayed by at least two decades the discovery that very low-carbohydrate diets (5-10%) can reverse the metabolic consequences of IRS.
Additional Reading

Professor T.D. Noakes (OMS, MBChB, MD, D.Sc., Ph.D.[hc], FACSM, [hon] FFSEM UK, [hon] FFSEM Ire) studied at the University of Cape Town (UCT), obtaining a MBChB degree and an MD and DSc (Med) in Exercise Science. He is now an Emeritus Professor at UCT, following his retirement from the Research Unit of Exercise Science and Sports Medicine. In 1995, he was a co-founder of the now-prestigious Sports Science Institute of South Africa (SSISA). He has been rated an A1 scientist by the National Research Foundation of SA (NRF) for a second five-year term. In 2008, he received the Order of Mapungubwe, Silver, from the President of South Africa for his “excellent contribution in the field of sports and the science of physical exercise.”
Noakes has published more than 750 scientific books and articles. He has been cited more than 16,000 times in scientific literature and has an H-index of 71. He has won numerous awards over the years and made himself available on many editorial boards. He has authored many books, including Lore of Running (4th Edition), considered to be the “bible” for runners; his autobiography, Challenging Beliefs: Memoirs of a Career; Waterlogged: The Serious Problem of Overhydration in Endurance Sports (in 2012); and The Real Meal Revolution (in 2013).
Following the publication of the best-selling The Real Meal Revolution, he founded The Noakes Foundation, the focus of which is to support high quality research of the low-carbohydrate, high-fat diet, especially for those with insulin resistance.
He is highly acclaimed in his field and, at age 67, still is physically active, taking part in races up to 21 km as well as regular CrossFit training.
References
- Noakes TD. It’s the insulin resistance, stupid: Part 1. CrossFit.com. 7 July 2019. Available here.
- Himsworth HP. Diabetes mellitus: Its differentiation into insulin sensitive and insulin insensitive types. Lancet 1(1936):127–130.
- Albrink MJ, Man EB. Serum triglycerides in coronary artery disease. Arch Intern Med. 103(1959): 4-8; Albrink MJ, Lavietes PH, Man EB. Vascular disease and serum lipids in diabetes mellitus: observations over thirty years (1931-1961). Ann Intern Med. 58(1963): 305-323. Albrink MJ, Meigs JW, Man EB. Serum lipids, hypertension and coronary artery disease. Am J Med. 31(1961): 4-23.
- Kuo PT. Hyperglyceridemia in coronary artery disease and its management. JAMA 201(1967): 87-94.
- Santen RJ, Willis PW, Fajans SS. Arteriosclerosis in diabetes mellitus. Correlations with serum lipid levels, adiposity, and serum lipid levels. Arch Intern Med. 130(1972): 833-843.
- West KM, Ahuja MMS, Bennett PH, et al. The role of circulating glucose and triglyceride concentrations and their interaction with other “risk factors” as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care 6(1983): 361-169.
- Carlson LA, Bottiger LE, Ahfeldt PE. Risk factors for myocardial infarction in the Stockholm prospective study. A 14-year follow-up focussing on the role of plasma triglycerides and cholesterol. Acta Med Scand. 206(1979): 351-360.
- Fontbonne AM, Eschwege EM. Insulin and cardiovascular disease: Paris prospective study. Diabetes Care 14(1991): 461-469.
- Fontbonne AM, Eschwege EM, Cambien F, et al. Hypertriglyceridemia as a risk factor of coronary heart disease mortality in subject with impaired glucose tolerance or diabetes: Results from the 11-year follow-up of the Paris prospective study. Diabetologia 32(1989): 300-304.
- Reaven G, Calciano A, Cody R, et al. Carbohydrate intolerance and hyperlipidemia in patients with myocardial infarction with known diabetes mellitus. J Clin Endocrinol Metab. 23(1963):1013-1023.
- Sohrade W, Boehle E, Bieglee R. Humoral changes in arteriosclerosis. Investigations on lipids, fatty acids, ketone bodies, pyruvic acid, lactic acid, and glucose in the blood. Lancet 2(1960): 1409-1416.
- Waddell WR, Field RA. Carbohydrate metabolism in atherosclerosis. Metabolism 9(1960): 800-806.
- Sowton E. Cardiac infarction and the glucose tolerance test. Brit Med J. 1(1962): 85-87.
- Wahlberg F. The intravenous glucose tolerance test in the atherosclerotic disease with special reference to obesity, hypertension, diabetic heredity and cholesterol values. Acta Med Scand. 171(1962): 1-7.
- Davidson PC, Albrink MJ. Insulin resistance in hyperglyceridemia. Metabolism 14(1965): 1059-1070.
- Reaven GM, Hill DB, Gross RC, et al. Kinetics of triglyceride turnover of very low density lipoproteins of human plasma. J Clin Invest. 44(1965): 1826-1833.
- Reaven GM, Lerner RL, Stern MP, et al. Role of insulin in endogenous hypertriglyceridemia. J Clin Invest. 46(1967): 1756-1767.
- Olefsky JM, Farquhar JW, Reaven GM. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 57(1974): 551-560.
- Farquhar JW, Frank A, Gross RC, et al. Glucose, insulin and triglyceride responses to high and low carbohydrate diets in man. J Clin Invest. 45(1966): 1648-1656.
- Coulson AM, Hollenbeck CB, Swislocki ALM, et al. Deleterious metabolic effects of high-carbohydate, sucrose-containing diets in patients with non-insulin-dependent diabetes mellitus. Am J Med. 82(1987): 213-220.
- Coulson AM, Hollenbeck CB, Swislocki ALM, et al. Persistence of hypertriglyceridemic effects of low-fat high-carbohydrate diets in NIDDM patients. Diabetes Care 12(1989): 94-101.
- Garg A, Bantle JP, Henry RR, et al. Effects of varying carbohydrate content of diet in patients with non-insulin-dependent diabetes mellitus. JAMA 271(1994): 1421-1428.
- Albrink MJ. Dietary and drug treatment of hyperlipidemia in diabetes. Diabetes 23(1974): 913-918.
- Goldberg RB. Lipid disorders in diabetes. Diabetes Care 4(1981): 561-572.
- Reaven G, Strom TK, Fox B. Syndrome X. The Silent Killer. The new heart disease risk. New York: Simon and Schuster, 2001.
- Bennett PH, Knowler WC, Pettit DJ. Longitudinal studies of the development of diabetes in the Pima Indian. In: Eschwege E, ed. Advances in diabetes epidemiology. New York: Elsevier Biomedical Press,1982; 65-74.
- Castelli WP, Doyle JT, Gordon T, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 55(1977): 767-772.
It’s the Insulin Resistance, Stupid: Part 2