Question: How might insulin resistance and impaired insulin signaling contribute to the development not only of diabetes but also dementia and other neurological disorders?
Takeaway: Based on a literature review conducted by a team of researchers from Curtin University in Australia, insulin resistance and impaired insulin signaling appear to contribute to the progression of both T2DM and AD, and it is well documented that reduced insulin action (whether by lack of release or by resistance to effect) figures centrally in the development and progression of both diseases.
Epidemiological and biological evidence have both highlighted the increased incidence of cognitive decline, global brain atrophy, and Alzheimer’s disease (AD) in patients with Type 2 diabetes (T2DM); recent research has sought to understand the common molecular bases connecting these diseases. Whether cause or consequence, inflammation, oxidative stress, amyloid-B accumulation, and mitochondrial dysfunction are present in AD and T2DM and appear to be plausible candidates for the molecular basis linking the two.
Chronic low-grade inflammation is a known component of both obesity and the metabolic syndrome. Macrophages that migrate into fat tissue secrete pro-inflammatory cytokines, such as tumor necrosis factor-𝛼 (TNF-𝛼) and interleukin-6 (IL-6), which have been shown to impair insulin signaling. Neutralizing them improves insulin sensitivity. Other pro-inflammatory cytokines can disrupt the pathways responsible for storing or oxidizing glucose and lipids, which impacts fat accumulation and the handling of sugar and leads to hyperglycemia and dyslipidemia. Hyperglycemia places an increased glucose burden on the beta cells of the pancreas as they work to secrete more insulin to restore normal blood sugar levels. A vicious cycle of inflammation breeding more inflammation ensues, and these inflammatory cytokines are known to be increased in obesity, T2DM, and AD.
A high-carbohydrate diet — the physiological consequence of which will be higher blood glucose, higher insulin secretion, and a greater rate of carbohydrate metabolism — can also result in increased generation of reactive oxygen species (ROS). ROS then can disrupt insulin signaling, bringing oxidative stress into the mix.
In the brain, insulin acts to control appetite, glucose levels, and lipid homeostasis. Insulin receptors are abundant throughout the brain but especially in the hippocampus, where they are known to modulate memory and learning and are necessary for synaptic plasticity. Recent research has shown reduced insulin levels in spinal fluid in the early stages of mild cognitive impairment and AD, and it’s known that reduced glucose metabolism in the brain occurs early in the course of AD. Prolonged high insulin levels reduce the transport of insulin across the protective blood brain barrier by downregulating insulin receptors.
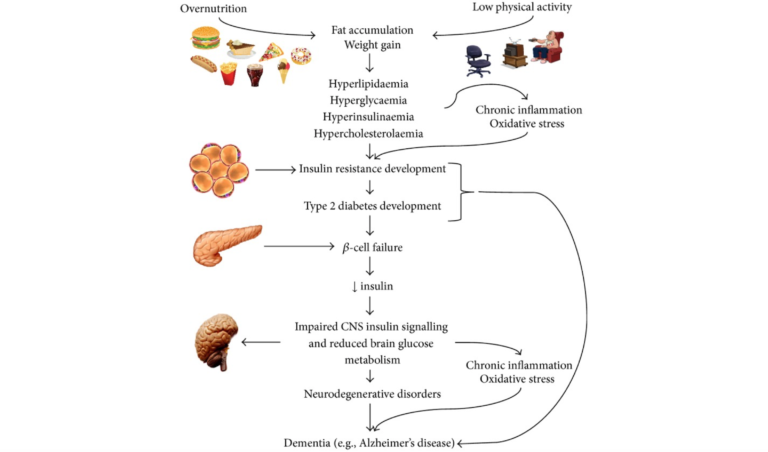
Key hallmarks of AD include brain atrophy (due to neuron cell death), deposition of amyloid plaques, accumulation of intracellular neurofibrillary tangles (NFTs), inflammation, and oxidative stress. The underlying mechanisms by which amyloid accumulates and its downstream events (NFTs and tau protein) develop have yet to be fully explained, but it is known that the accumulated amyloid protein can inhibit autophosphorylation of the insulin receptor and alter a number of cellular processes that result in neuron dysfunction, including insulin signaling. It’s also clear insulin loses some of its effectiveness in the AD brain.
This article’s review of the literature works carefully through common molecular pathways linking disturbed glucose metabolism, oxidative stress, inflammation, and the accumulation of advanced glycation endproducts (AGEs) as they contribute to the progression of both AD and T2DM.
Comments on Inflammation and Oxidative Stress: The Molecular Connectivity Between Insulin Resistance, Obesity, and Alzheimer’s Disease
Isn't the slang term for Alzheimer's these days 'Type 3 Diabetes'. Seemingly for good reason.
👍👍👍!!!
On the image, there is a term "Hypercholesterolaemia". I don't yet see the connection in the text. Usually we see that high cholesterol is not the problem. On the other hand high amounts of LDL seems do be a bad thing. And we have learned in earlier posts that messured LDL could potentially be Lp(a) instead. Can anyone enlighten me where is the connection in this text / respectively what produces LDL and Lp(a)? According to Wikipedia (on hypercholesterolaemia) its animal fats more than plant fats, but I don't think I should believe that.
By the way, I would like to hear more on gut bacteria. If there is a good point other than ethics in a vegan diet (in a well balanced vegan diet), than to me it is a better gut flora aka non-vegans should eat more veggies to better this (shouldn't a better gut flora reduce the production of Trimethylamine oxide even if one does not reduce meat intake?) etc.
Sorry, when I seem to be uninformed. But this one term in the image popped in my eyes, and I could not find it in the text.
I think you are the first person in an internet comment section not claiming to know and be right about everything. Kudos to you sir. I wish I could answer your initial question but I cannot. I agree with your desire to hear more about gut flora, my limited understanding and searching indicates that the science is still really new in that area and CrossFit seems to be trying hard to put out as concrete of information as possible so it may be a while. Purely anecdotal but my wife and I worked closely with the yoga community for many years (high proportion of Vegans) and I have never met a group of people with more nagging gut issues and little irritations and spells of temporary illness popping up here and there regularly, conversely, in the CrossFit gyms we've worked in the only instances of clients talking about gut issues have been vegetarians or clients still transitioning from the SAD diet. Grass fed steak and greens just seems to work, don't really need to know why (black box) just that it does.
I noticed that hypercholesterolaemia was listed in this process too. But I believe it is listed as an outcome of fat accumulation and weight gain rather than necessarily being causal of downstream effects.
Lp(a) is a subclass of LDL particle produced in the liver (that unfortunately is not assessed by the standard "lipid panel" that most doctors do which just determines total LDL ). Whether you have a lot of Lp(a) or not, is genetically determined. If you had one parent who had high Lp(a), you have a 50% chance of having it as well.
https://www.lipoproteinafoundation.org/ to learn more.
High Lp(a) is an independent risk factor for cardiovascular disease, whereas for most people high LDL is not (except for cases such as familial hypercholesteremia (FH)).
Because Lp(a) is poorly cleared by the body and circulates in the blood for a long time relative to plain old LDL, the cholesterol in it is more likely oxidized, thereby making it more atherogenic. My guess is that oxidative stress such as that described in this article might accelerate that process.
Look for work by Thomas Dayspring or Chris Masterjohn for all you ever wanted to know about lipids.
Thank you, guys. And thanks for the further resources, I will definitely give it a read.
I guess, and like being corrected, that LDL delivers fats to the cells. And HDL delivers fat back to the liver. So, more LDL means more fat storage and more HDL means using fat as energy (extremely simplified). Insulin controls those processes. So, a nonfunctional metabolism disturbs lipoproteins. (that’s the connection to the text I guess).
Black Box is great, but I’m still curious if one can do more to prevent atherosclerosis. How can someone reduce Lp(a)? How can someone reduce radicals? How can someone reduce trimethylamine oxide?
The movie “Game Changers” (which still leaves me angry for watching it) advocates cutting meat for exactly this. But I think of most people; they go to a Burger Chain and order a Burger, Fries with Coke. Cutting Meat means cutting the most and only healthy food from this plate. That’s where the movie goes wrong. But the movie got me more curious about atherosclerosis.
However, Crossfit style workouts and Crossfit style Nutrition seems to be THE SOLUTION. Guess I am looking for shortcuts.
Inflammation and Oxidative Stress: The Molecular Connectivity Between Insulin Resistance, Obesity, and Alzheimer’s Disease
7