Increasing interest in the metabolic theory of cancer suggests metabolic health or sickness may play a role in the cause and progression of the disease. Here, David Sabatini and Peggy Hsu review the significance of a variety of metabolic defects in cancer vis-à-vis the Warburg effect. They write:
Described decades ago, the Warburg effect of aerobic glycolysis is a key metabolic hallmark of cancer, yet its significance remains unclear. In this Essay, we re-examine the Warburg effect and establish a framework for understanding its contribution to the altered metabolism of cancer cells.
As a tumor expands, a variety of oncogenes also linked to metabolism (Ras, Akt, Myc, p53) are upregulated, each impacting glucose uptake and/or glycolysis. While the balance of these oncogenes varies by cancer, the dysregulated metabolism observed in many cases can be explained by shifts in them.
These metabolic alterations support accelerated growth and reproduction by increasing the availability of cellular building blocks. They also protect the rapidly growing cells by reducing reactive oxygen species production.
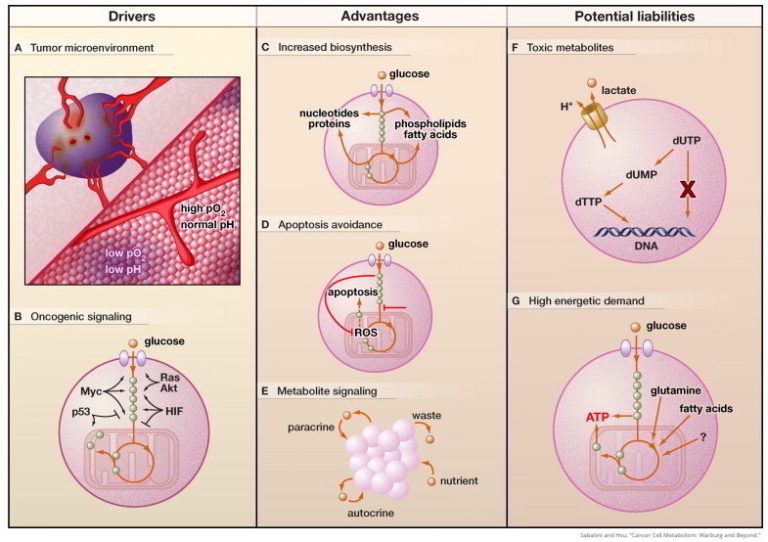
Figure 1: The Altered Metabolism of Cancer Cells Drivers (A and B).
The authors note we need to look beyond glucose to fully understand the metabolic dysfunction present in cancer:
We emphasize the need to explore beyond a glucose and energy-centric driven model of cancer metabolism to a broader one that encompasses all of the metabolic needs of a cancer cell. Perhaps it is time to step out from under Warburg’s shadow.
Comments on Cancer Cell Metabolism: Warburg and Beyond
Looking forward to more on the Warburg effect
Sadly, what they say is true. "Many mysteries remain unsolved in our understanding of even normal human metabolism, let alone that of cancer cells."
Cancer Cell Metabolism: Warburg and Beyond
2