In this October 2018 editorial in Frontiers in Psychiatry, Michael Hengartner and Martin Plöderl argue trials investigating the impact of antidepressants show statistically significant but not clinically significant effects, and even these meager effects may be eroded by known biases.
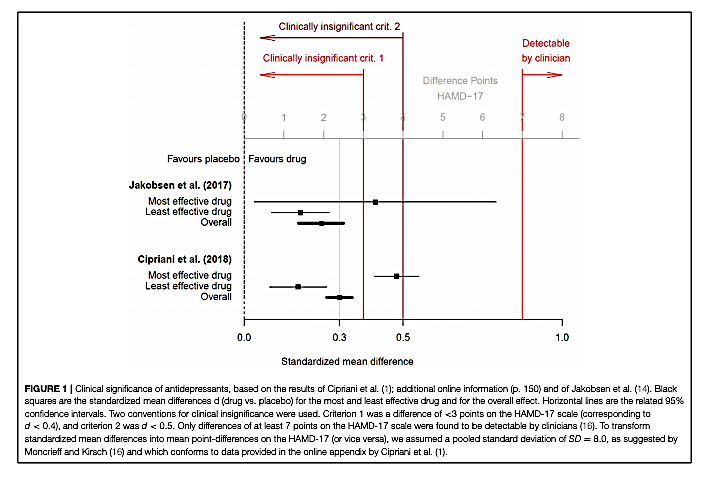
The authors present the data from two recent meta-analyses summarizing the impact of antidepressant medications on measures of clinical depression (1). Both meta-analyses found antidepressants had statistically significant benefits compared to placebo, but both also found a small mean effect size, equal to or below d = 0.3. Hengartner and Plöderl note this small mean effect size is similar to the effect size observed in previous meta-analyses, as cited by A. Cipriani (1), and corresponds to an improvement of approximately 2 points on the Hamilton Depression Rating Scale (HAM-D 17).
The meta-analyses authors and others have argued improvements in HAM-D scores of less than 3 points (equivalent to an effect size of d = 0.5) are clinically irrelevant, and improvements of less than 7 points are not clinically detectable (2). By this standard, Hengartner and Plöderl argue the mean benefit associated with antidepressant treatments is below the threshold for clinical significance.
Even this small effect size, Hengartner and Plöderl argue, may be inflated due to widely recognized biases that exaggerate the perceived impact of antidepressant drugs in clinical trials. Blinding is difficult in antidepressant trials, as trial staff and even patients can often detect placebo use by a lack of side effects; when active placebos are used that provide the same side effects as antidepressants, the effect size shrinks (3). Additionally, trials that have assessed patients’ own perception of benefit have shown smaller effects than those relying on clinicians’ assessment of benefit (4).
These and other possible biases, combined with the small baseline effect size, have led some to argue bias accounts for the majority of the perceived benefits of antidepressants in clinical trials (5).
Importantly, these uncertain benefits come with certain costs, as previously discussed on CrossFit.com. Antidepressants have been associated with increased risk of dementia, stroke, obesity, and all-cause mortality. These side effects may be a worthwhile trade-off for a subset of patients who benefit from antidepressants, but the evidence suggests the majority of patients experience benefits that are of little to no clinical significance.
References
- Cipriani A, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018 Apr 7;391(10128): 1357-1366.
- Moncrieff J, Kirsch I. Empirically derived criteria cast doubt on the clinical significance of antidepressant-placebo differences.
Contemp Clin Trials. 2015 Jul;43: 60-2. - Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression.
Cochrane Database Syst Rev. 2004;(1): CD003012.
- Barbui C, Furukawa TA, Cipriani A. Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re-examination of published and unpublished data from randomized trials. CMAJ. 2008 Jan 29;178(3): 296-305.
- Gøtzsche, P. Why I think antidepressants do more harm than good. Lancet 1.2 (July 2014): 104-6.
Comments on Statistically Significant Antidepressant-Placebo Differences on Subjective Symptom-Rating Scales Do Not Prove that the Drugs Work: Effect Size and Method Bias Matter!
Interesting piece. The treatment of depression is certainly an area where much improvement is needed and these results, if true, are disappointing and point to the need for improved treatment modalities. On the other hand, I wonder if “clinically detectable” is an appropriate measuring stick for the effect of a drug? Is it possible that an effect can be clinically *undetectable* yet still meaningful with respect to the course of the disease? Notably, the sensitivity and specificity of the Hamilton Depression Rating Scale are 86.4% and 92.2%, respectively (according to the Psychiatry & Behavioral Health Learning Network). Though, certain populations show less diagnostic power (e.g., sensitivity and specificity of post-stroke depression were found to be 78.1% and 74.6%, respectively per https://www.sciencedirect.com/science/article/abs/pii/S0033318202703666).
Finally, the discordance between perceived benefits from the patient versus clinician perspectives underscores the influence of bias, perhaps indicating how one's role in the care process can influence their interpretation of treatment. Note that the Hamilton Depression Rating scale is observer-rated (not patient-rated).
I applaud the authors for continuing to investigate the efficacy of these treatments after a strong statement like Dr. Pariante from the Royal College of Psychiatrists saying the Cipriani et al. study, “finally puts to bed the controversy on antidepressants, clearly showing that these drugs do work in lifting mood and helping most people with depression,” (as stated in the Frontiers article referenced in this post).
Statistically Significant Antidepressant-Placebo Differences on Subjective Symptom-Rating Scales Do Not Prove that the Drugs Work: Effect Size and Method Bias Matter!
1