Reprinted with permission from an article published on Medium.com on Oct. 17, 2018.
A case report in 2000 described a 24-year-old woman presented to her physicians with some unusual symptoms. During exercise, she experienced a full grand mal seizure, without any previous history of epilepsy. In the prior 6 months, she had become very tired and noted episodes of trembling, blurred vision, and confusion. She could control these symptoms by eating something.
Upon investigation, she was found to suffer from a rare insulin secreting tumor of the pancreas known as an insulinoma. This tumor was massively overproducing insulin inappropriately, causing her blood sugar to fall very low, going down as far as 1.6 mmol/L after an overnight fast. She had way too much insulin in her system.
Upon further questioning, she also noted acne, hirsutism and her periods had become very irregular, varying from 40 to 44 days over the previous year. An ultrasound revealed polycystic ovaries, and bloodwork revealed high testosterone levels, which gave her a diagnosis of PCOS. She had a normal body mass index and was not overweight.
A scan revealed a two-centimeter tumor mass in her pancreas, and she underwent an uneventful surgery to remove it. Four months after her successful operation, her menstrual cycles became regular at 28 days, she lost 4 kg of weight (8.8 pounds), and her acne and hirsutism fully resolved. Bloodwork revealed that her insulin level had normalized, and along with it, her testosterone levels.
This case provides dramatic insight into the causal role of excessive insulin in PCOS as well as weight gain. Earlier studies had already hinted at its importance. High insulin levels are reported in 75% and 30% of obese and non-obese women. As insulin levels rise, so do rates of obesity, irregular menstrual cycles and blood testosterone levels. In the study of diseases, the most crucial piece of information is the etiology, a medical term for what causes it. If you do not understand what causes the problem, it is very difficult to design therapies that have a reasonable chance of success. The link with obesity suggests that excessive insulin may play a role in the development of PCOS.
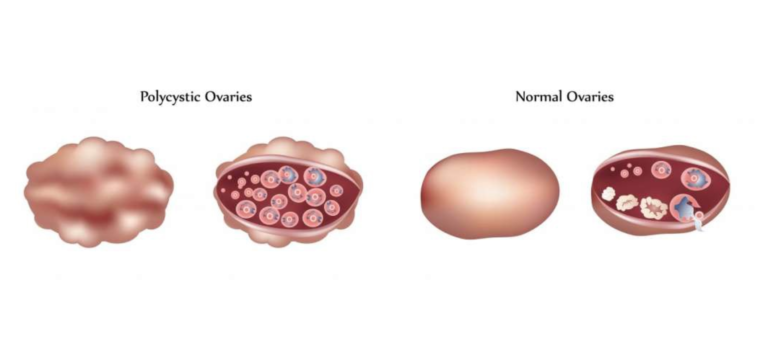
Consider the three main symptoms of PCOS:
- Hyperandrogenism
- Menstrual irregularity
- Polycystic ovaries
The hormonal underpinnings of PCOS began to be appreciated in the 1950s, with the development of the radioimmunoassay that could measure various hormone levels. In the 1960s and 1970s, research focused on luteinizing hormone (LH) and follicle stimulating hormone (FSH), key regulators of the normal menstrual cycle. For many years, an abnormal LH/FSH ratio was considered diagnostic of PCOS. By the 1980s, testosterone was recognized as the main androgen that was responsible for the majority of problems.
Testosterone is produced normally in both the ovaries and the adrenal gland — a small gland sitting on top of the kidneys. In addition to androgens, the adrenal gland also produces other hormones, including cortisol, adrenalin, and aldosterone.
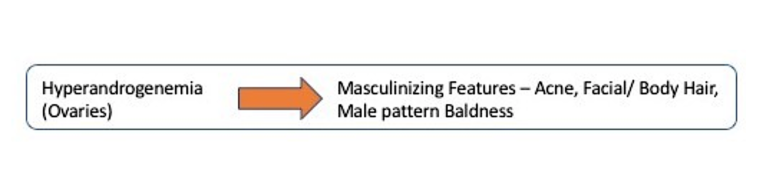
Once we recognized that excessive androgen production caused most of the symptoms of PCOS, the next step was to figure out which organ was responsible for the overproduction. There were only two possibilities — the ovaries or the adrenal glands. In the normal situation, the ovaries and adrenal glands contribute equally to testosterone production. By 1989, studies showed that in women with PCOS the ovary is the key source of excessive testosterone production. Specifically, it is the theca cells within the ovary that are responsible for the overproduction of hormones.
One more line of evidence implicated the ovaries as the source of excess testosterone in PCOS. The initial modern description of PCOS by Stein and Leventhal had also revealed that surgically removing a wedge of the ovary (ovarian wedge resection) often restored normal ovulation and normal menstrual cycles. This is only possible if the source of excess androgens is the ovary. After all, if the adrenal gland was responsible for overproducing androgens, cutting a little wedge of the ovary out would not make any difference. The hyperandrogenism of PCOS secreted mostly by the ovaries caused the symptoms of hirsutism and infertility.
Direct measurement of blood testosterone levels is not part of the diagnostic criteria and is highly problematic for three reasons. First, blood levels vary widely throughout the day and with age and menstrual status. Secondly, even in established PCOS, the ovarian portion of T production only rises to about 60% of total daily production of T. Third, a major contributing factor to the excess androgen effect seen in PCOS (hirsutism, acne, etc.) is not excessive testosterone but rather low levels of sex hormone binding globulin (SHBG).
Hormones do not circulate freely. Instead, they travel around the bloodstream bound up with other proteins that accompany them to their proper destination. SHBG is the protein designed to work with testosterone. Imagine you are traveling around New York City for a business meeting. It’s difficult to get to your destination on foot. It’s slow and as you walk around, you get tempted to shop at stores along the way. You’d never get to your intended destination. So instead, a better solution is to travel around New York City inside a taxi, which brings you directly to your meeting. The same thing happens in our bodies.
Hormones travel around the bloodstream accompanied by transport proteins. Otherwise, the unchaperoned “free” hormones would stop off at every tissue to exert their effects and never get to their intended destination. The testosterone produced in the ovary might stop by the liver, kidneys, and fat tissue before getting to its intended destination of the skin. So, a better solution is to carry the hormones with binding proteins that transport them directly to the target organ. This is true for most hormones in the human body.
These hormone-carrying proteins are very specific for each hormone. Thyroid hormone, for example, is only carried by thyroid-binding globulin (TBG). The term globulin refers to a protein that is globular in shape, which many of these carrier proteins are. Thyroid hormone can only hitch a ride on its proper globulin (TBG) but cannot bind to, say, sex-hormone-binding globulin (SHBG), which carries testosterone.
What happens if the proper carriers are not available? Imagine you are in New York City just as a baseball game in Yankee Stadium finishes. You find yourself with 50,000 other fans on the street and there are insufficient taxis. All the fans are just milling around the street going into all the stores and bars. It’s overcrowded around Yankee Stadium, but the places where these fans are going (home) are all empty.
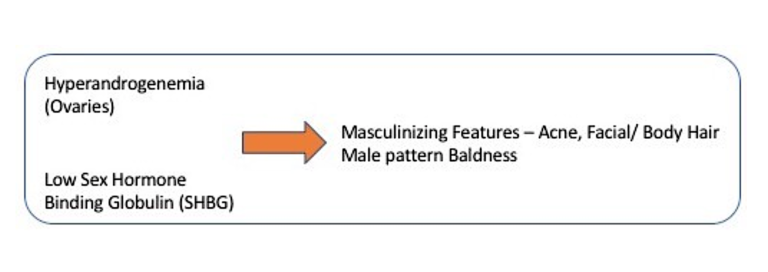
The same thing happens in our body. If there are not enough carrier proteins like SHBG, then testosterone floats around freely in the blood, just like the fans wandering the streets around Yankee Stadium. Testosterone, in high levels, starts to exert its masculinizing effects on the neighboring organs because it is not getting to its proper destination. Thus, you develop acne, excessive facial hair, and male pattern baldness. The total amount of testosterone might be the same, but the lack of carrier protein SHBG allows excessive effect of these androgens.
Hyperinsulinemia
Women with PCOS have low levels of the carrier proteins SHBG that bind the testosterone. This amplifies testosterone’s effect, even if levels are not particularly high. But what causes this lack of SHBG? The culprit is too much insulin.
Insulin is the major regulator of SHBG production in the liver. The higher the insulin, the lower the SHBG production. This relationship holds true not just in women, but also in men. Decreasing insulin levels through weight loss increases SHBG production.
A population based study in Sweden confirmed the inverse relationship. Type 1 diabetics, with very low insulin levels, had very high SHBG. Type 2 diabetics, with very high insulin, had very low SHBG. Insulin directly reduces liver production of SHBG and therefore increases the levels of free and bioavailable androgens. Thus, excessive insulin causes both:
- overproduction of testosterone
- decreased SHBG levels that leads to increased testosterone effect
As far back as 1980, the striking correlation between blood levels of insulin and testosterone was noted. Insulin and testosterone blood levels showed an astounding 85% correlation to each other. These two hormones were moving in almost exact lockstep, which is highly suggestive that one hormone was directly stimulating the other.
Did high testosterone cause high insulin, or did high insulin cause high testosterone? Elegant studies with isolated cell cultures clarified this connection. When you purify ovarian cells and bathe them in insulin, they increased testosterone production significantly. The opposite is not true. If you bathe cells in testosterone, nothing happened, since the ovary does not produce insulin. The pancreas is the organ responsible for insulin production and secretion. If you give testosterone to experimental subjects, insulin secretion by the pancreas does not change at all. Clearly, insulin drove testosterone production and not the other way around.
Human studies have confirmed that high insulin indeed increases androgen levels. Direct insulin infusion measurably increases the levels of androgens. The more insulin you give, the higher the testosterone production in the ovary. Even 24 hours after the insulin infusion was stopped, the testosterone levels continued to be elevated. The ovary itself is particularly rich in insulin receptors, which seems rather strange at first glance. Insulin is a hormone most commonly associated with digestion, blood glucose, and body fat. Why would the ovaries carry insulin receptors? In fact, the pathways that link reproductive function and metabolism are seen in virtually every animal in existence. This is an evolutionarily conserved trait found in everything from fruit flies to roundworms to human beings. Why?
The answer is that all animals need to know that food is available before committing to reproduction. Raising children requires a good deal of resources including adequate food supplies for both the expectant mother and the developing baby. Adults can survive on relatively low levels of food energy and nutrients. During World War II, for example, many people survived on what would now be considered woefully inadequate amounts of food.
Adults don’t need to grow and therefore require fewer nutrients. Human livers or lungs, for example, do not grow once they reach adult size. In tough times, we can break down old liver cells to build new liver cells. A baby, however, must eat enough nutrients to build new liver cells. A baby may enter the world weighing only 7 pounds but eventually grows to perhaps 150 pounds. In addition to energy for normal cellular function, it must also gain 143 pounds of matter to build proteins, fat cells, internal organs, muscles, etc. This is a very resource-intensive labor of love.
If humans produced too many babies at a time when food is scarce, neither the baby nor the mother would survive. Therefore, the ovary must have reliable information about the availability of food in the outside world. It should only produce eggs at a time that food is available to sustain the growth of the baby. How is this poor ovary supposed to know about the outside world, trapped inside the pelvis, with no eyes, ears, or nose of its own?
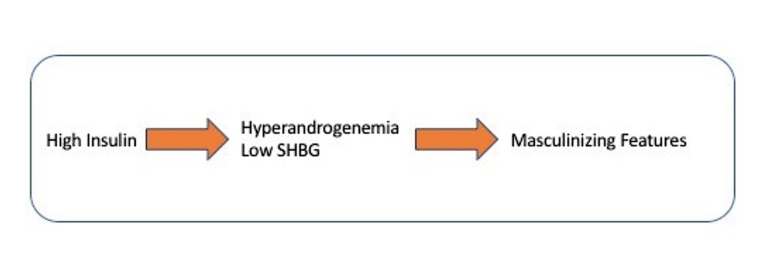
The solution is to develop nutrient sensors that signal ample food availability. Insulin is one of those nutrient sensors. If you eat food, insulin rises, a signal that food is available. The ovary’s insulin receptors pick up this fact and proceed along normal development of the human egg. But if too much insulin is available, then the process goes awry.
High insulin increases testosterone and decreases SHBG, thereby causing the masculinizing features. Blocking insulin decreases testosterone levels and can be demonstrated experimentally with a drug called diazoxide. Lowering insulin with other medications such as metformin or TZDs also has the same effect of lowering testosterone levels. In patients with PCOS, surgical removal of part of the ovaries reverses the hyperandrogenism but not the high insulin levels.
It has become increasingly clear that a high insulin level is the primary factor stimulating excessive ovarian production of testosterone. This increased androgen (hyperandrogenism) is responsible for the masculinizing features of PCOS, including acne and hirsutism. This is a great start to understanding the etiology of PCOS. But there are two other main features to consider. Is insulin responsible for the lack of ovulation and polycystic ovaries, too? Stay tuned.
Comments on What Causes PCOS?
This article is much appreciated. I've been doing a lot of research on PCOS and endometriosis over the years. I'm still trying to figure out the probability of misdiagnosis of PCOS vs. endometriomas. I'm assuming the presentation is similar including the symptoms. Just wanted to know someone else's opinion.
Very interesting article.
I'm interested in more of these findings?
Quick question on this is could Hypothyroidism be a misdiagnosis instead of PCOS? Also what is the recommended treatment for PCOS?
Very interesting, also interested in further findings in this research.
What Causes PCOS?
3