Breathing, or ventilation, is one part of the picture of how we get oxygen into the blood and carbon dioxide out of the blood. Ventilation draws fresh air into the lungs before gas exchange and expels it after gas exchange. During gas exchange, the second part of the picture, the body exchanges one gas for another — in this case, the gases involved are oxygen and carbon dioxide. This exchange occurs at two locations: at the alveoli, where oxygen is picked up and carbon dioxide is removed, and at the systemic circulation’s capillary interface with cells (at a muscle cell for example), where oxygen is removed and carbon dioxide is picked up. How this is accomplished is conceptually simple but also exquisitely intricate and complex when considered in detail.
The air we inhale from the environment has a fairly consistent composition, and it’s not all oxygen:
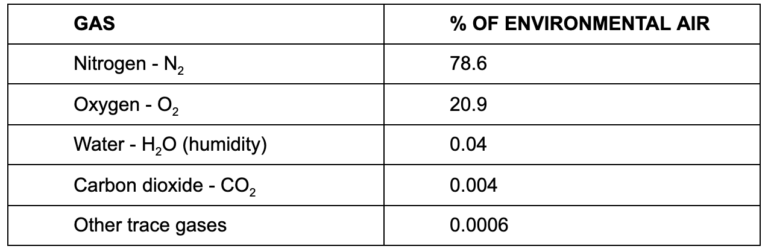
Nitrogen is inert and a non-factor in our simplistic explanation here, as are atmospheric water vapor and other trace gases. Oxygen and carbon dioxide are the active ingredients, and oxygen is by far more prevalent in environmental air than carbon dioxide.
Gases move from areas of high pressure to low pressure. It’s why a carbonated beverage can or bottle hisses when opened. The contents are at a higher pressure inside the container, and when the seal is broken some carbon dioxide gas (or nitrogen in some products) dissolved in the beverage inside moves out of solution rapidly and ejects into the air around the can. Over time the carbon dioxide dissolved in the can will drop to the same level as in atmospheric air (0.004%) and the process will end; the beverage “goes flat.”
The atmosphere has a basic pressure of 760 mmHg. Think of this as barometric pressure in weather reports. Air is the mixture of gases described above, with each making up some fraction of the total. Each gas present in the mixture we call environmental air contributes to the total pressure (760 mmHg) relative to the quantity of that gas present. The fraction of total pressure provided by the individual gas is called partial pressure (approximates):
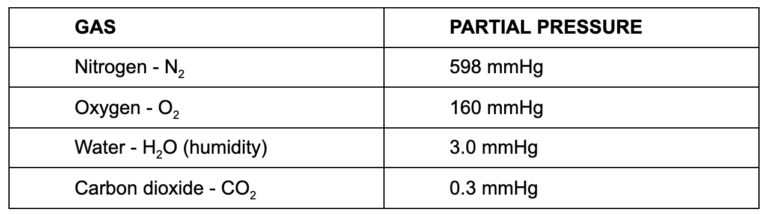
So, if we consider the first major player, oxygen, any area with lower than a 160 mmHg partial pressure of oxygen will draw atmospheric oxygen into that low pressure area. For example, inside an alveoli, the partial pressure of oxygen is about 105 mmHg. This is far less than that of atmospheric air, and as a result the pressure gradient (160 > 105) drives the movement of oxygen out of the air and into the alveoli.
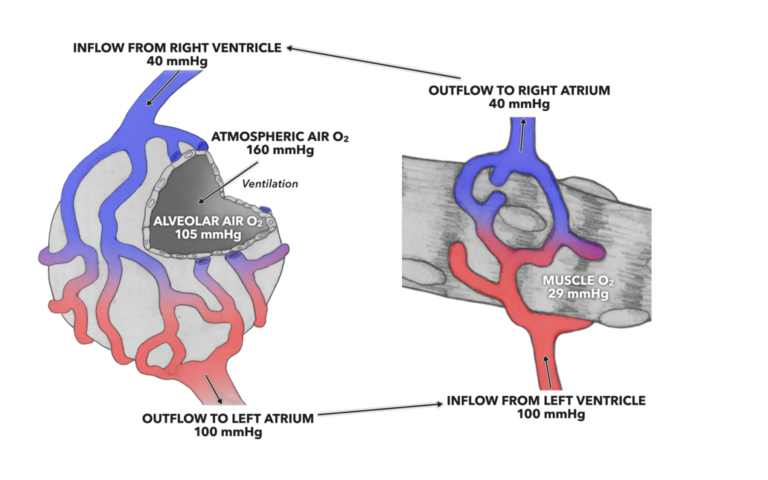
Figure 1: The pressure gradient for oxygen at the alveoli and muscle drives diffusion across the respiratory membrane from the alveolus to the pulmonary capillaries and from systemic capillaries into muscle.
There is an intermediary here that we have to consider: blood. The gases needed for life are carried by and dissolved by blood, and it is the partial pressures therein that facilitate gas exchange. When blood exits the pulmonary alveolar capillary bed, the partial pressure of oxygen is 100 mmHg. That partial pressure is present until the opposite set of gas exchange processes happen at the capillaries within the systemic circulation.
If we consider the partial pressure of oxygen within a muscle cell, we find that it is about 29 mmHg. This sets up a concentration gradient between capillary blood and the muscle cell (100 > 29) and pushes the oxygen out of the capillaries and into the muscle cell.
If we make similar considerations for carbon dioxide, the pressure gradient is opposite to that seen in the oxygen pressure gradient. Inside the alveoli, carbon dioxide’s partial pressure is 5.2 mmHg, and that pressure is by far greater than the 0.3 mmHg found in atmospheric air. In this instance the pressure gradient between atmospheric air and the alveoli (0.3 < 5.2) drives carbon dioxide out of the alveoli into the air. At the muscle, the partial pressure present causes movement of carbon dioxide out into the systemic capillaries.
At this point we need to think about what creates this lower intracellular oxygen concentration. Recollect that cellular metabolism requires oxygen and produces carbon dioxide. If we only consider the derivation of biological energy from a single sugar molecule or a simple fat molecule, we see that lots of oxygen gets consumed and lots of carbon dioxide gets produced:
One C6H12O6 + six O2 + two ATP > yields > six CO2 + six H20 + thirty eight ATP
C = carbon
H = hydrogen
O = oxygen
C6H12O6 = the simple sugar glucose
CO2 = carbon dioxide
ATP = adenosine triphosphate (biological energy)
This is just a single metabolic reaction set working 24/7/365 in the human body. There are well over 500 such reaction series working anabolically or catabolically, all directly or indirectly consuming oxygen and producing carbon dioxide. Combined, these reactions set up the partial pressure gradients at the systemic cell/systemic capillary interface required for gas exchange.
Another chemical feature aids in this process as well. Most people will already know that hemoglobin, a big globular protein in red blood cells, picks up oxygen as it passes through pulmonary capillaries and holds onto it until passing through systemic capillaries. Inside the muscle cell, there is another big globular protein that has an even higher affinity for oxygen: myoglobin. As oxygen offloads from the hemoglobin in the red blood cells within the capillaries into the muscle cell interior, myoglobin hoovers up the new oxygen molecules, keeping the pressure gradient going until all binding sites are full. At the end of this process, the blood will exit the systemic capillary bed, and this is where we began: with this blood destined for the pulmonary circulation and the alveolar capillary beds, ready to start another round of gas exchange.