In 1972 and 1973, a large National Heart, Lung, and Blood Institute (NHLBI) grant funded the Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT) alongside the Multiple Risk Factor Intervention Trial (MRFIT) to substantiate the then-emerging understanding of modifiable heart disease risk factors.
The trial randomized 3,806 hypercholesterolemic men to cholestyramine resin (a bile acid sequestrant) or placebo for an average of 7.4 years. Both groups were told to reduce dietary cholesterol, total fat, and saturated fat.
The treatment and control groups both showed significant diet-related reductions in cholesterol (11.1 mg/dL in the treatment group, 12.6 mg/dL in controls over the first 2 months of the trial), with the cholestyramine group showing an additional decrease in cholesterol compared to controls (41.8 mg/dL in year 1 and 23.3 mg/dL by year 7). Both groups also showed an increase in triglyceride levels, with little impact on HDL; LDL cholesterol changes paralleled total cholesterol changes.
Table
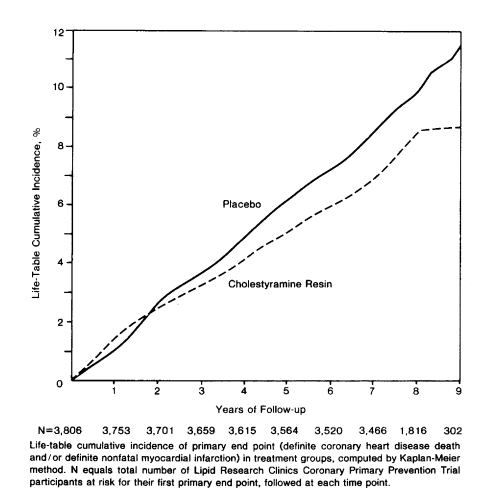
Over 7 years, there were 187 definite heart attacks in the treatment group compared to 155 in the controls—a statistically significant 19% relative risk reduction. There was a smaller reduction in coronary mortality (38 versus 30 deaths in control versus treatment) and no impact on total mortality (71 versus 68 total deaths).
The authors’ original conclusions reflect these results and offer a cautionary note regarding our limited ability to extrapolate from these results to support other cholesterol-lowering interventions:
The LRC-CPPT demonstrated that treatment with cholestyramine resin reduced the incidence of [coronary heart disease] CHD. This result is in agreement with those of previous clinical trials of cholesterol lowering, which have shown a general trend of efficacy for selected CHD end points. However, the earlier trials have not been regarded as conclusive because of such factors as inadequate sample size, absence of a double blind, failure to achieve identical treatment groups, inadequate cholesterol lowering, or questionable statistical procedures.
The authors add: “Caution should be exercised before extrapolating the CPPT findings to cholesterol-lowering drugs other than bile acid sequestrants.”
Unfortunately, this reservation was lost in subsequent discussions, and results were used not long after to support the claim that any 1% reduction in cholesterol will reduce heart attack risk by 2%, whether the cause derives from a pharmaceutical intervention or diet.
Comments on The Lipid Research Clinics Coronary Primary Prevention Trial
All the answers were present but somehow no one paid attention to them.
The Lipid Research Clinics Coronary Primary Prevention Trial
1