There is a saying in law that one should never ask a question of a witness without knowing the exact response the witness will provide.
Similarly, if one uses science to market one’s products, as does Gatorade through its GSSI, then the absolute rule is that one must never fund a study without knowing the results before the study begins, because there is always the risk that the results may undermine the false science one uses to promote one’s products. In their ignorance, Gatorade and the GSSI funded three studies that did just that. And one of these studies faithfully reproduced all the conditions necessary to explain why Dr. Cynthia Lucero and other female runners developed fatal EAHE during various U.S. marathons between 1993 and 2002.
Not that their favored scientists came to that conclusion when they reported those studies in the scientific literature. But it does not take much probing to discover the true meaning of these three studies.
Study 1
Despite clear evidence that the amounts of sodium present in sports drinks such as Gatorade cannot prevent EAH and EAHE in those who drink either “to stay ahead of thirst” or to ensure they do not lose any weight during exercise, in 2008 Baker et al. (1) continued their industry-funded attempts to discover a beneficial role for the sodium present in sports drinks. They introduced their study thus:
Replacing sweat electrolyte losses, particularly during/after prolonged exercise in the heat, may play an important role in the prevention of fluid and electrolyte imbalances. Profuse sweating can lead to significant fluid and electrolyte losses, and there are some indications that Na+loss is a contributing factor in the etiology of heat-related whole body muscle cramps (2, 3). Additionally, excessive electrolyte loss may contribute to the development of exercise-associated hyponatremia during particularly long events. (4, 5)
Hopefully all readers will now realize that these statements are false and disproved by the studies we have reviewed in this series. Interestingly, all the authors cited (2-5) in Baker et al.’s publication enjoy very tight relationships with Gatorade and the GSSI. These authors do not seem to recognize any work that arises from outside the Gatorade/GSSI cohort.
The authors studied eight subjects who ingested three beverages with different sodium concentrations at four different rates that would produce four different body-weight changes at the end of exercise. The four weight groups included the following: no weight change, a weight gain of 2%, and weight losses of -2% and -4%. The drinks contained either 0,18, or 30 mmol/L of sodium. In order to achieve these different weight changes, subjects drank either ~4.0, 2.6, 1.0, or 0.2 liters while performing seven bouts of 15 minutes of running (105 minutes of exercise) with two minutes of rest between runs. It is probable that only the 1.0-liter intake (500 mL/hr) was truly “ad libitum.” The most important findings of the study were not, unsurprisingly, what the authors reported.
First, my analysis (6) of their data found that there was a significant linear relationship with a negative slope between the post-exercise blood sodium concentrations and the extent of the net electrolyte (sodium and potassium) deficit generated during exercise (Figure 1; left panel).
This relationship was the opposite of what was expected since the highest post-exercise blood sodium concentrations were measured in the groups with those who had developed the largest net electrolyte losses (Group A in Figure 1, left panel), whereas the second-lowest concentrations occurred in subjects who did not develop an electrolyte deficit during exercise (Group B in Figure 1, left panel).
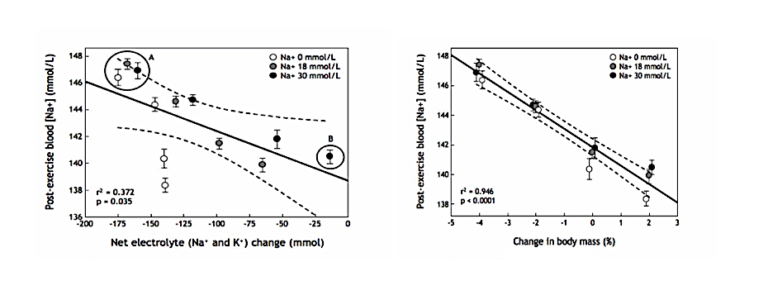
Figure 1: Blood sodium concentrations in the same subjects when they drank three different solutions at four different rates during exercise to produce four different levels of electrolyte (sodium and potassium) deficits (left) and weight change (right). Left panel: There was a significant linear relationship with a negative slope between the post-exercise blood sodium concentrations and the net electrolyte (sodium and potassium) change produced by different rates of sodium ingestion during exercise. But the relationship was the reverse of what was expected so that the three groups with the highest post-race blood sodium concentrations had the largest net electrolyte deficits (Group A), whereas the group with the second-smallest deficit had one of the lowest post-exercise blood sodium concentrations (Group B). Right panel: There was a highly significant linear relationship with a negative slope between the post-exercise blood sodium concentration and the change in body mass during exercise. As a result, 95% of the variance in the blood sodium concentrations in this experiment was explained by variation in body mass during exercise (Reproduced from reference 6).
These findings disprove, in the most elegant manner, the simplistic USARIEM model (5) for how a sodium deficit causes EAH. Instead, they again confirm that the blood sodium concentration is homeostatically regulated during exercise and that overdrinking (in the presence of SIADH) is the most important factor that disturbs homeostasis.
Clearly, the body is quite able to protect the blood sodium concentration, even when there is a substantial sweating-induced sodium deficit (Figure 1; left).
The importance of this study is perhaps that this biological law, which we had proposed in 1985 (7) and proved in 1991 (8), had now been confirmed by research funded by the sports drink industry and directed by Dr. Lindsay Baker, an employee of PepsiCo, the manufacturer of Gatorade.
However, Baker and her colleagues of course could not sanction that conclusion. Instead, they quoted two other industry-funded studies by Vrijens and Rehrer (9) and Twerenbold et al. (10), which apparently showed that Gatorade can prevent a fall in blood sodium concentrations during exercise. So, Baker et al. concluded, “Sodium ingestion becomes even more critical as the duration of exercise increases” (p. 97).
This conclusion is even more remarkable because another study by Baker et al. (11) had already shown that Gatorade did not prevent a fall in blood sodium concentrations among those encouraged to drink “ahead of thirst” to prevent any weight loss during exercise. As I will show, that study faithfully recreated in the laboratory the conditions that would have explained why Lucero died of EAHE in the 2002 Boston Marathon.
Nevertheless, Baker et al. apparently did not feel it necessary to mention that in both the cited studies (9, 10), subjects were required to overdrink and gain weight during exercise in order to show a fall in blood sodium concentrations. Indeed, in Vrijens and Rehrer’s study (9), the factor that prevented this fall in blood sodium concentrations was vigorous diuresis (increased urine production), indicating an immediate and sustained suppression of antidiuretic hormone (ADH) secretion in response to overdrinking (12).
Significantly, when the data from Baker et al.’s study are interpreted by someone with no link to the industry, it becomes clear that those data neatly disprove the authors’ conclusions. If anyone honestly believes that the nature of one’s employment does not influence the conclusions one is allowed to draw in scientific studies, this study should disabuse us all of that particular fairy tale.
A final point is that Baker et al.’s study (1) was published in the Journal of Applied Physiology, which one would assume is a fiercely independent publication.
However, the editors of that journal resolutely refused to publish our critique of the paper. Our critique ultimately found an academic home in the British Journal of Sports Medicine (6).
Why would the Journal of Applied Physiology not wish to publish our alternative analysis, which is so clearly valid (Figure 1; right panel)?
Perhaps because the publishers of that journal receive or have received funding from Gatorade and the GSSI?
Study 2
Another industry-funded study was designed to show that “hypovolemic hyponatremia” and “also dehydration” can develop in “athletes with unusually high sweat sodium concentrations concomitant with large sweat volume losses” (13, p. 864). The study evaluated a group of women with a history of developing hyponatremia, usually EAH. The athletes were studied during exercise while they received a variety of different hormonal interventions, including treatment with the female hormones oestradiol and progesterone and an antagonist to GnRH, the chemical messenger from the brain (hypothalamus) that stimulates the ovaries’ production of female hormones.
The study was funded by Gatorade and the GSSI, and also assisted in the career advancement of Dr. Nina Stachenfeld, who studies the effects of reproductive hormones on cardiovascular regulation, thermoregulation, and fluid regulation in women. Perhaps there was some subtle interest in encouraging her loyalty to the Gatorade brand.
Stachenfeld became a member of the committee that drafted the 2007 ACSM Position Stand on Exercise and Fluid Regulation (14). With funding from Gatorade and the GSSI, she may have been more likely to agree with the consensus opinions of the other five members of that expert committee who had close connections with either Gatorade and the GSSI (Drs. Burke, Eichner, and Maughan) or the USARIEM (Drs. Sawka and Montain).
The primary goal of the study was to determine whether hormonal effects would “increase water retention and sodium loss in women at high risk for EAH” (p. 864). Subjects exercised for three hours but drank for only the final two hours of the study.
During the first hour of exercise, their blood sodium concentrations rose regardless of the nature of the hormonal intervention (Figure 2; left panel). But once the subjects began to drink (at a rate of >0.9 – 1.1 L/hr), their blood sodium concentrations began to fall, with the greatest fall occurring in women with a history of EAH. In some, the fall was large enough to force the scientist to terminate the experiment in order to prevent any subjects from developing EAHE.
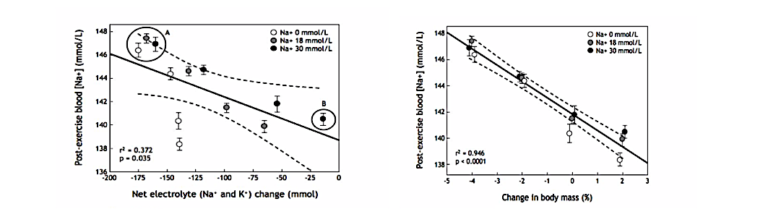
Figure 2: Blood sodium concentrations among subjects in the Stachenfeld study (13) rose for the first 60 minutes of exercise when subjects did not drink (left panel). But when they drank to a prescribed regimen in excess of thirst, those with a history of hyponatremia showed a progressive fall in blood sodium concentrations, which caused some to develop EAH. The fall in blood sodium concentrations was due to a progressive increase in the plasma (blood) volume (right panel), which was greater in subjects with a history of hyponatremia but unrelated to the hormonal intervention.
This fall was unrelated to the hormonal intervention. Sweat and urine sodium losses were also the same in both groups under all conditions; thus, differences in sweat and urinary sodium losses could not explain why only the group with a history of hyponatremia developed EAH.
Instead, most (but not all) of the differences were due to greater water retention in the vascular space in subjects with a history of hyponatremia (Figure 2; right panel).
The authors concluded, “Water retention was the primary contributor to the lower serum sodium concentrations in (women with a previous history of hyponatremia)” (p. 864). They continued: “Our data point to excessive water drinking relative to a smaller body size as a primary cause of hyponatremia in women susceptible to it” (p. 871).
This conclusion seems rather similar to the conclusions we drew in 1985 (7), proved in 1991 (8), and proved again in 2005 (15).
Study 3: Gatorade and the GSSI inadvertently find explanation for Lucero’s death
I was inspired to write Waterlogged (16) in order to present the evidence that if the biology of EAHE is properly understood, no athlete ever needs to die from it again. That book, along with this series of columns for CrossFit.com, shows that the promotion of overdrinking by scientists and sports medicine organizations with a too-cuddly relationship with the sports drink industry was the ultimate cause of the global epidemic of EAH and EAHE that began after 1981.
It is perhaps appropriate to conclude this series of columns with the ultimate proof of this: a laboratory study (11) funded by Gatorade/GSSI that accurately reproduced the conditions necessary to produce EAH and EAHE in female runners who take more than five hours to run/walk a 26.2-mile marathon. The study was completed in a laboratory directed by Dr. W. Larry Kenney, who has enjoyed a long and warm friendship with Gatorade, the GSSI, and the ACSM. He was elevated to president of the ACSM in 2003 and 2004.
For the study, 27 healthy “older” subjects, aged between 54 and 70 years, exercised according to a rather unusual protocol in which they were active for 15 minutes and then spent 15 minutes at rest while staring at a 700-mL bottle containing either distilled water or Gatorade. Apparently, subjects “were not encouraged to drink but were told that more fluid was available if needed” (p. 791). The subjects were also apparently unaware that their fluid consumption was being monitored (p. 791).
Despite the absence of any apparent pressure to drink, subjects managed to achieve heroic rates of fluid ingestion: 1.1 liters when they ingested Gatorade and 0.86 liters when they ingested water. As a result, subjects gained weight during the experiment, but the weight gain was greater in women than men. Blood sodium concentrations fell, especially in the women, during the experiment; average blood sodium concentrations fell in the total sample of subjects, and the extent of the fall was very similar whether water or Gatorade was ingested (Figure 3). Any small difference is of no biological significance.
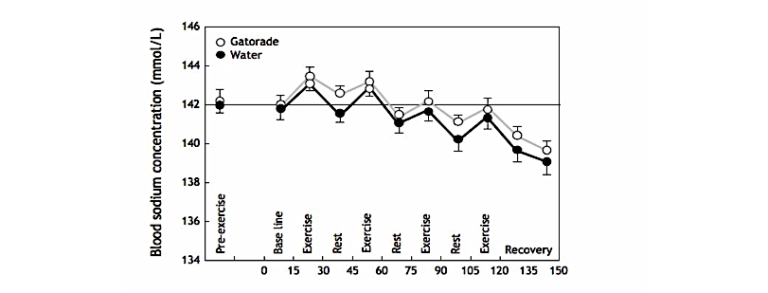
Figure 3: The study of Baker et al. (11) found that subjects who gained weight during exercise because they ingested either water or Gatorade at rates in excess of their rates of weight loss (sweat) developed a progressive fall in blood sodium concentrations whether they drank water or Gatorade.
One female subject who was the most heroic drinker developed EAH when she drank either Gatorade (2.8 liters, causing her blood sodium concentration to fall to 131 mmol/L) or water (2.7 liters, dropping her blood sodium concentration to 126 mmol/L) (Figure 4).
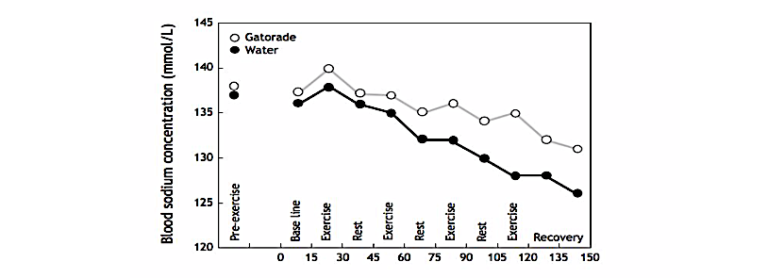
Figure 4: A 65-year-old female subject developed symptomatic EAH when she drank 2.7 liters of water during the 150-minute experiment; her blood sodium concentrations fell to “only” 131 mmol/L when she drank Gatorade, at which concentration she was apparently without any symptoms.
Naturally, the authors concluded that when given free access to fluid and plenty of time in which to drink (so that the exercise time equalled the potential drinking time), subjects “drank enough to match sweating rates and maintained their body mass.” On the unproven and likely false grounds that any weight loss during exercise impairs exercise performance and increases the risk of heatstroke, they further concluded that drinking so much was beneficial since it prevented any weight loss during exercise. Furthermore, the ingestion of Gatorade was superior to water since the plasma volume increased when Gatorade was ingested. Since the study was funded by the sports drink industry, it is perhaps understandable why the real findings of the study were never properly discussed — until now.
For example, the experimental design was bizarre and clearly developed to ensure that subjects overdrank and hence developed EAH when they drank water. The hope would have been that under those conditions, the ingestion of Gatorade would prevent the development of EAH.
Unfortunately, the study proved the opposite: The ingestion of Gatorade does not prevent a progressive fall in blood sodium concentrations in those who gain weight during more prolonged exercise. But this concern was not raised in the published manuscript, which suggests this is an example of peer-review bias in a study published in Medicine and Science in Sports and Exercise (MSSE), the premier scientific publication of the ACSM.
Subsequently, I sent a letter to MSSE in which I argued that those data confirm our 1985 hypothesis that “some subjects provided with an unlimited supply of palatable fluids cannot regulate their blood sodium concentrations during exercise. Thus blood sodium concentrations fell equally in men and women who drank sufficiently to prevent ‘voluntary dehydration’”(17, p. 193).
I also pointed out that the authors appeared “unusually biased” toward the value of sports drinks, noting the authors’ conclusion that ingesting a sports drink was a superior choice since only a sports drink can produce weight gain and an increase in plasma volume during exercise. But since there is no evidence that weight gain during exercise is beneficial, “perhaps an opposite conclusion would have been reached if the water bottling industry had funded this study; specifically, that water is the superior fluid since its ingestion reduces the risk of overdrinking during exercise” (p. 193).
My doctoral student at the time, Jonathan Dugas, decided also to write a letter to critique the paper (18). The letter was not considered for publication by MSSE on the grounds that the journal does not publish separate letters from the same laboratory that raise issues about the same published article. Thus, the letter had to be sent to the British Journal of Sports Medicine. Dugas made the following points:
- “The authors failed to conclude that the consumption of any hypotonic fluid at these rates nevertheless causes a steady decline in serum sodium concentrations. Instead Baker et al. (11) concluded that a (sports drink) better restores plasma volume and is therefore a more effective fluid replacement in older adults during intermittent exercise” (p. 372).
- “This conclusion ignores the fact that, in their trial, serum sodium concentrations in both the men and women fell during exercise, regardless of the type of fluid they were ingesting, and that a falling blood sodium concentration will eventually lead to hyponatraemia and its associated symptoms and medical complications.”
Dugas then provided a figure, reproduced here (Figure 5), in which he re-plotted the data from Figure 4 to show the expected time course of the fall in blood sodium concentrations in the 65-year-old female subject who developed EAH. He concluded that if she had continued to drink Gatorade at that rate, she would have developed EAHE after between five and six hours.
He did not add that the authors had reproduced the precise experimental conditions that would explain why Lucero had developed EAHE after five hours of running in the 2002 Boston Marathon (16; p. 6-9).
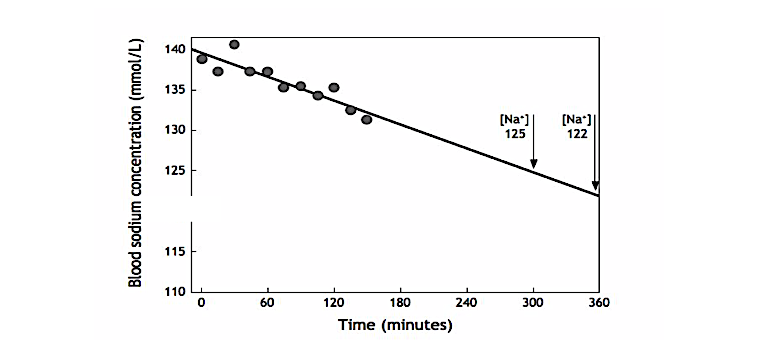
Figure 5: When Dugas plotted the data for blood sodium concentrations in the female subject who developed EAH in the study by Baker et al. (11, Figure 4), he showed that had she continued to drink Gatorade at the same rate that she sustained for the 150 minutes of that study, she would have reached a blood sodium concentration of 125 mmol/L after five hours and 122 mmol/L after six hours, exactly as happened to Lucero’s blood sodium concentration in the 2002 Boston Marathon. Thus, the experiment had neatly recreated the exact mechanism leading to Lucero’s death.
Not unexpectedly, this letter unleashed a state of apoplexy in Gatoradeland, and Kenney was quick to respond (19). While he “appreciated” the letter, he found Figure 5 “scientifically puzzling” since it presumed that the subject would continue to overdrink for 5-6 hours until she collapsed from EAHE. Rather, in his opinion, “negative feedback characteristics inherent in physiology … would have prevented this (petite older female) subject from continuing both exercise and her overdrinking behavior long before 6h.”
But if such “negative feedback characteristics inherent in physiology” are so powerful, then Lucero and many others would not have died and there would have been no need for Waterlogged (16) and this series of CrossFit columns.
Moreover, if Kenney and his colleagues do indeed believe that humans respond appropriately to “negative feedback,” why has it been necessary for the ACSM/GSSI/Gatorade to inculcate athletes’ minds with the idea that they cannot rely on other physiological “feedback characteristics” such as thirst? Why, over the past two decades, have Kenney and his colleagues advised athletes to ignore the “feedback characteristics” of thirst on the (false) grounds that drinking to thirst is not sufficient to ensure health during exercise?
Instead, the positive encouragement to overdrink and the fear of catastrophic consequences such as “dangerous dehydration” have been so pervasive that these “negative feedback characteristics” have no hope. They are simply ignored by those who, like Lucero, may be terrified of becoming dehydrated or dying from heatstroke (even when they exercise in frigid conditions in which heatstroke is a remote possibility).
Kenney used scientific gobbledygook in his attempt to bury the crucial importance of the evidence from the study by Baker et al. Perhaps if he was more independent of the Gatorade, GSSI, and ACSM, his response would have been different. Perhaps he may have shown more concern for those athletes who stand to suffer from decades of erroneous drinking advice.
Instead, he should have admitted that in his laboratory he had recreated the very circumstances that caused the deaths of Lucero and other female marathon runners in the U.S. (20).
He should have emphasized how important it is for the ACSM, GSSI, and Gatorade to understand the critical relevance of this finding if they wished to prevent more avoidable deaths from EAHE in the future.
Conclusion
Between 2005 and 2009, three separate industry-funded studies (1, 11, 13) showed that sodium balance has no impact on the extent to which blood sodium concentration falls during exercise and that abnormalities in the regulation of body water content (Figure 1, right panel; Figure 2, right panel) explain the development of EAH and EAHE. We had already established such findings in 1991 (8).
Gatorade and the GSSI had very effectively demonstrated that the sodium present in their product provides no measurable benefit for exercisers. At the same time, they also proved that the campaign to promote high rates of fluid consumption during exercise — the Zero-Percent Dehydration Doctrine (21) — led to grim consequences, producing the global epidemic of EAH and EAHE.
It’s finally time to start telling the truth.
Additional Reading
- The Hyponatremia of Exercise, Part 1
- The Hyponatremia of Exercise, Part 2
- The Hyponatremia of Exercise, Part 3
- The Hyponatremia of Exercise, Part 4
- The Hyponatremia of Exercise, Part 5
- The Hyponatremia of Exercise, Part 6
- The Hyponatremia of Exercise, Part 7
- The Hyponatremia of Exercise, Part 8
- The Hyponatremia of Exercise, Part 9
- The Hyponatremia of Exercise, Part 10
- The Hyponatremia of Exercise, Part 11

Professor T.D. Noakes (OMS, MBChB, MD, D.Sc., Ph.D.[hc], FACSM, [hon] FFSEM UK, [hon] FFSEM Ire) studied at the University of Cape Town (UCT), obtaining a MBChB degree and an MD and DSc (Med) in Exercise Science. He is now an Emeritus Professor at UCT, following his retirement from the Research Unit of Exercise Science and Sports Medicine. In 1995, he was a co-founder of the now-prestigious Sports Science Institute of South Africa (SSISA). He has been rated an A1 scientist by the National Research Foundation of SA (NRF) for a second five-year term. In 2008, he received the Order of Mapungubwe, Silver, from the President of South Africa for his “excellent contribution in the field of sports and the science of physical exercise.”
Noakes has published more than 750 scientific books and articles. He has been cited more than 16,000 times in scientific literature and has an H-index of 71. He has won numerous awards over the years and made himself available on many editorial boards. He has authored many books, including Lore of Running (4th Edition), considered to be the “bible” for runners; his autobiography, Challenging Beliefs: Memoirs of a Career; Waterlogged: The Serious Problem of Overhydration in Endurance Sports (in 2012); and The Real Meal Revolution (in 2013).
Following the publication of the best-selling The Real Meal Revolution, he founded The Noakes Foundation, the focus of which is to support high quality research of the low-carbohydrate, high-fat diet, especially for those with insulin resistance.
He is highly acclaimed in his field and, at age 67, still is physically active, taking part in races up to 21 km as well as regular CrossFit training.
References
- Baker LB, Lang JA, Kenney WL. Quantitative analysis of serum sodium concentration after prolonged running in the heat. J Appl Physiol. 105(2008): 91-99.
- Stofan JR, Zachwieja JJ, Horswill CA, et al. Sweat and sodium losses in NCAA football players: a precursor to heat cramps? Int J Sport Nutr Exerc Metab. 15(2005): 641-652.
- Bergeron MF. Heat cramps during tennis: a case report. Int J Sport Nutr. 6(1996): 62-68.
- Hiller WD. Dehydration and hyponatremia during triathlons. Med Sci Sports Exerc. 21(1989): S219-S221.
- Montain SJ, Cheuvront SN, Sawka MN. Exercise associated hyponatraemia: quantitative analysis to understand the aetiology. Br J Sports Med. 40(2006): 98-105.
- Noakes TD. Changes in body mass alone explain almost all of the variance in the serum sodium concentrations during prolonged exercise. Has commercial influence impeded scientific endeavour? Br J Sports Med., 2010.
- Noakes TD, Goodwin N, Rayner BL, et al. Water intoxication: a possible complication during endurance exercise. Med Sci Sports Exerc. 17(1985): 370–375.
- Irving RA, Noakes TD, Buck R, et al. Evaluation of renal function and fluid homeostasis during recovery from exercise-induced hyponatremia. J Appl Physiol. 70(1991): 342-348.
- Vrijens DM, Rehrer NJ. Sodium-free fluid ingestion decreases plasma sodium during exercise in the heat. J Appl Physiol. 86(1999): 1847-1851.
- Twerenbold R, Knechtle B, Kakebeeke TH, et al. Effects of different sodium concentrations in replacement fluids during prolonged exercise in women. Br J Sports Med. 37(2003): 300-303.
- Baker LB, Munce TA, Kenney WL. Sex Differences in Voluntary Fluid Intake by Older Adults during Exercise. Med Sci Sports Exerc. 37(2005): 789–796.
- Noakes TD. The hyponatremia of exercise, part 9. CrossFit.com. 12 May 2019. Available here.
- Stachenfeld NS, Taylor HS. Sex hormone effects on body fluid and sodium regulation in women with and without exercise-associated hyponatremia. J Appl Physiol. 107(2009): 864-872.
- Sawka MN, Burke LM, Eichner ER, et al. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc. 39(2007): 377-390.
- Noakes TD, Sharwood K, Speedy D, et al. Three independent biological mechanisms cause exercise-associated hyponatremia: Evidence from 2,135 weighed competitive athletic performances. PNAS 102(2005): 18550-18555.
- Noakes TD. Waterlogged: The serious problem of overhydration in endurance sports. Champaign, IL: Human Kinetics, 2012.
- Noakes TD. Sports drinks: prevention of “voluntary dehydration” and development of exercise-associated hyponatremia. Med Sci Sports Exerc. 38.1(2006): 193.
- Dugas J. Sodium ingestion and hyponatraemia: Sports drinks do not prevent a fall in serum sodium concentration during exercise. Br J Sports Med. 40(2006): 372.
- Kenney WL, Baker LB. Over‐extrapolation of research. 19 Apr 2006. Available here.
- Noakes TD. The hyponatremia of exercise, part 8. CrossFit.com. 2 May 2019. Available here.
- Noakes TD. The hyponatremia of exercise, part 6. CrossFit.com. 10 April 2019. Available here.
Comments on The Hyponatremia of Exercise, Part 12
Excellent series, Dr Noakes. Thank you for continuing to shine the light of Truth.
Every time I read one of the hyponatremia of exercise part I learn a little more but more importantly I realize that I have so much is conception coming from the industry pushing some wrong conclusion to some false study!
Thanks for opening our eyes.
Excellent series. Please correct fig. 2, it is now a duplicate of fig. 1.
I read it all yesterday, and found Taubes' writing on salt politics today, i.e. the persistent advice to reduce salt intake.
This series illustrates salt homeostasis clearly; excess is being sweat / uriated out, and fast. It would be tempting to connect the dots towards
salt intake in general...
Another tempting direction is analogue of cholesterol homeostasis. Most of it is hidden in cells, but the (arbitrary) blood value limit keeps falling -thus needing fee-based treatment. And of course hypertension...
Hell of a series. Thanks for bringing this to light.
Thanks Troy. It's a privilege to be able to use this medium to write in an unconstrained manner to expose examples of how modern science is, and has been, corrupted for commercial gain. And how scientists and scientific organisations can and do become willing partners in this deceit.
Thanks especially to Greg Glassman CEO for reaching out to me many years ago when he read my book Waterlogged and told me how he appreciated my stand to tell the truth. That led me to investigate the CrossFit phenomenon and ultimately to try it for myself. With great results.
My experience suggest to me that the combination of the LCHF diet with 2 or more hours' CrossFit exercise per week is the best basis for long term health. Of course there are many other additions that one needs to take care of, but without this specific combination of nutrition and exercise, the rest will be less beneficial.
I agree with Mr Glassman's view that we cannot reform medicine from the inside. All we can do is to educate the users of medicine to differentiate that which is glorious and highly effective about the profession from that which serves the medical-pharmaceutical-scientific industries' desire to maintain us as long-term fee-paying captives to their definition of "health".
CrossFIt Health pursues a quite different definition of health and provides the perfect vehicle for many of us to bring our renegade (disruptive) views on medicine and science to the public attention without the fear of censorship.
Thanks again for that sense of freedom and purpose, Mr Glassman!
The Hyponatremia of Exercise, Part 12
5