Question: Is pancreatic fat increased in diabetics, independent of other factors?
Takeaway: Pancreatic fat is more than twice as high in diabetics as in similar subjects matched for BMI and other factors. The findings of this study support the hypothesis that pancreatic fat accumulation contributes to β-cell dysfunction in diabetics.
Type 2 diabetes is marked by progressive β-cell dysfunction (1). Two causes of β-cell dysfunction have been widely proposed: hyperglycemia (and associated glucose toxicity) and chronic exposure to elevated fatty acid levels (2). As insulin resistance develops and the total mass of adipose tissue in the body increases, circulating fatty acid levels rise; over time, when fatty acid supply exceeds oxidation (utilization), fatty acids begin to accumulate beyond the fat cells (3).
This 2007 cross-sectional study compared pancreatic fat levels in 12 diabetic subjects to 24 nondiabetics with similar age, BMI, and waist circumference. The diabetics, as expected, had elevated HbA1c, fasting glucose, fasting insulin, and fasting triglycerides, and had lower fasting HDL levels than the nondiabetics.
Mean pancreatic fat content was more than twice as high in diabetic subjects as nondiabetic subjects (20.4% vs. 9.7%). Across all subjects, and across nondiabetic subjects specifically, higher levels of pancreatic fat were associated with higher HOMA-IR, as well as impaired β-cell glucose sensitivity (4, 1).
In diabetic subjects, increased pancreatic fat did not further impair any measure of glucose tolerance or insulin sensitivity, which implies that once a threshold of pancreatic fat burden is reached, additional fat storage does not further impair β-cell function.
β-cell glucose sensitivity, the parameter most closely associated with pancreatic fat levels, has previously been shown to be a reliable predictor of diabetes development and progression (5).
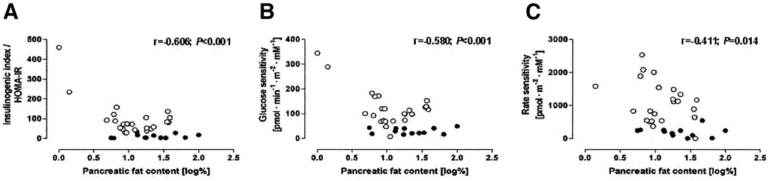
Association between pancreatic fat content and HOMA-IR, glucose sensitivity, and insulin secretion rate in diabetic (black dots) and nondiabetic (white dots) men; among nondiabetic men, elevated pancreatic fat content is associated with impaired glucose-mediated insulin response.
Interestingly, there was no association between hepatic fat content or overall visceral fat content and either pancreatic fat content or β-cell function. Diabetics had higher levels of hepatic (liver) fat than nondiabetics (16.6% vs. 7.7%), and this elevated liver fat was associated with increased fasting plasma glucose, fasting triglycerides, and circulating fatty acid levels. While previous research has shown a relationship between visceral fat and pancreatic β-cell function, this relationship has been inconsistent and may merely be the result of a confounding relationship between increased visceral fat and impaired whole-body insulin sensitivity.
It is not yet understood why and how increased pancreatic fat impairs insulin response (6). Triglyceride accumulation within the β cell may directly lead to an inflammatory state that impairs insulin production and secretion (7). Alternatively, the same factor may impair microvascular function and so reduce effective glucose exposure to or insulin release by the β cell. High levels of circulating fatty acids also correlate with high levels of adipokines, which may directly damage the β cell (8).
Overall, these results indicate pancreatic β-cell fat content, independent of BMI and waist circumference, is significantly increased in diabetic subjects. These results are consistent with previous work indicating elevated liver fat is associated with the metabolic syndrome and adds support to the overall hypothesis that organ-specific fat accumulation, due to its effect on insulin sensitivity and glucose tolerance, directly predicts and may play a causal role in the development of metabolic distress.
Pancreatic Fat Content and β-Cell Function in Men With and Without Type 2 Diabetes