Response of the Sports Drink Industry to Proof That Exercise-Associated Hyponatremic Encephalopathy (EAHE) Is not Due to Sodium Deficiency and Cannot Be Prevented by Drinking Electrolyte-Containing Sports Drinks
Like everyone else at the time, I had absolutely no idea why the lady who wrote to me in June 1981 had become the world’s first case of EAHE (1). In particular, I could not answer her question about why her blood sodium concentration had fallen by more than 20 mmol/L during the seven hours that she had run in that year’s Comrades Marathon. So, in my response letter to her, I meekly suggested that perhaps she had lost an inordinate amount of salt in her sweat and developed a sodium deficiency. I also speculated that she may have retained some fluid as well.
In time (2), we established that at least three abnormalities must be present for EAHE to develop.
First, there must be voluntary overdrinking. Overdrinking during exercise began to be promoted after 1982 through acceptance of the fallacious Water as a Tactical Weapon Doctrine (3), which led to the American College of Sports Medicine’s (ACSM) Zero-Percent Dehydration Doctrine (4).
Second, there must be abnormal fluid retention. It took all of us at least 10 years to appreciate that the normal response to voluntary overdrinking during exercise should simply be to excrete all the fluid ingested in excess of that which is required to balance sweat losses. Clearly, if athletes are finishing ultramarathon races with a fluid excess of more than 12 lb./6 kg (5) and take hours or, more commonly, days to excrete that excess, then something we hadn’t considered was happening.
(Consider that the kidneys filter 7.5 liters of blood every hour but reabsorb 99.4% of that fluid. But by reabsorbing just 90%, the kidneys could correct a 12-lb./6-kg fluid excess in about six hours—which is exactly what happens with the correct management of EAHE with hypertonic (3-5%) saline solutions given intravenously, as described in a previous column).
We later realized that the culprit is an abnormal secretion of antidiuretic hormone (ADH). ADH is one of the most powerful hormones in the body; at very low blood concentrations, it prevents urine production. It does this by maximizing water reabsorption by the kidneys. Persons who develop EAH or EAHE secrete ADH inappropriately and have the syndrome of inappropriate ADH secretion (SIADH)—“inappropriate” because ADH secretion is stimulated by dehydration and should be completely inhibited by overhydration. But in persons with SIADH, increasing levels of overhydration fail to inhibit ADH secretion. Instead, continuing secretion of ADH in the face of sustained overdrinking produces progressive fluid retention, leading to swelling of the brain, which, if not corrected timeously, leads to death from respiratory failure.
Third, there must be movement of sodium from the blood into an internal sodium store, likely in bone and cartilage. In the 1950s, it was well accepted that sodium, which is normally thought to exist exclusively outside the cells—in the bloodstream and fluid between the cells but not within the cells—must also be stored within cells, the so-called osmotically inactive exchangeable sodium stores (6).
When a group of us interested in EAH and EAHE combined our data on blood sodium and body weight changes in 2,153 athletes competing in endurance events (2), we observed a very interesting finding, shown in Figure 1.
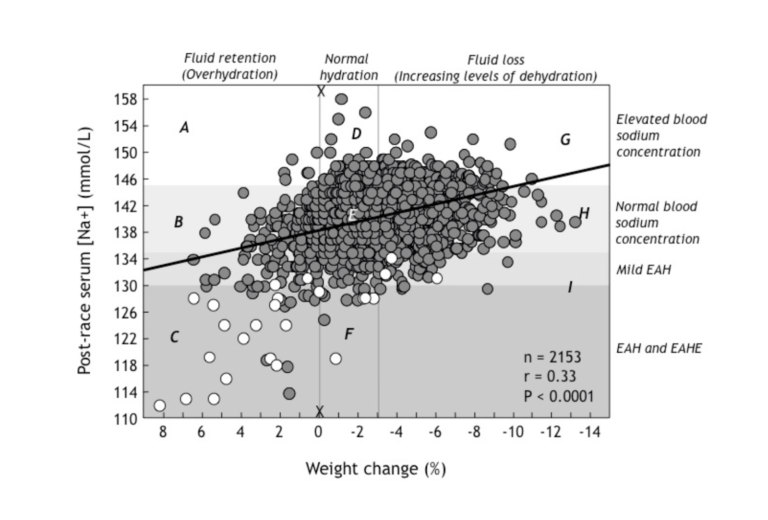
Figure 1:Post-race serum (blood) sodium concentrations rise (moving from left to right in this figure) with increasing levels of weight loss (dehydration) in 2,153 athletes competing in a variety of different endurance sporting events. Thus, dehydration (right side of the figure) causes the blood sodium concentration to rise, whereas fluid retention due to SIADH and overdrinking associated with removal of sodium into the osmotically inactive exchangeable sodium stores (left side of figure) causes the blood sodium concentration to fall, reaching values below 130 mmol/L (EAH) in those who show a weight gain of between +0-9%. Open dots indicate athletes with EAHE. As section C of the figure demonstrates, in general, these were athletes who had gained the most weight during these events (Reproduced from reference 24, p. 288).
The most interesting thing to observe in Figure 1 is that blood sodium concentrations vary widely at any level of weight change. This indicates that individuals differ, sometimes dramatically, in their responses to the same degree of weight loss or weight gain.
Take, for example, the vertical line X-X. This shows that for the same degree of post-race hydration (in this case no or little weight loss during exercise), the blood sodium concentration can vary from 118 mmol/L to 158 mmol/L.
Since we know that this difference is not due to differences in either levels of hydration or external sodium losses from the body (5), it can be due only to changes in the distribution of sodium within the body. Athletes with very high blood sodium concentrations therefore must have added some sodium into the bloodstream from the osmotically inactive exchangeable sodium stores, with the reverse happening in those with very low post-race blood sodium concentrations.
Thus, it is extremely likely that the relocation of sodium from the blood into the osmotically inactive exchangeable sodium stores within cells contributes significantly to the very low post-race blood sodium concentrations measured in subjects in section C in the figure.
Conversely, the ability of some athletes to maintain normal blood sodium concentrations in the face of large weight gains (section B in Figure 1) would be due to the addition in the blood of sodium originating from the intracellular exchangeable sodium stores.
Finally, there was no evidence that sodium deficiency developed in persons with EAH or EAHE. Instead, we showed that sodium losses were the same in those who finished ultramarathon races with or without EAH or EAHE (5).
In summary, what these studies found was that overhydration is the key preventable abnormality in the development of EAH and EAHE whereas excessive sodium losses play no part (although relocation of sodium within different body compartments is clearly involved). Prevention of the condition therefore requires that athletes be warned not to overdrink during prolonged exercise. Since the condition is not due to sodium deficiency, overdrinking a sodium-containing sports drink will not prevent EAH or EAHE.
Consequences of Our Finding for the Sports Drink Industry
Evidence that the promotion of excessive fluid consumption during exercise is the direct cause of EAH and EAHE posed some ethical challenges for the sports drink industry and the sports medical front organizations that it funds and uses to protect its marketing dogmas from objective scientific scrutiny. Should they all come clean and admit that the guidelines they had actively promoted were quite wrong and not evidence-based? This would be an unwise commercial decision and especially damaging for the credibility of a product that was supposedly based on “science” (7).
An alternative short-term solution would be to spread misinformation and doubt about the real “truth” of any scientific information that a specific industry finds uncomfortable. The technique is described in the book Doubt Is Their Product (8).
The second option would be simply to ignore our definitive findings—which is the route they chose.
For example, neither of the 2007 ACSM Position Stands, one on exercise and fluid replacement (9) and the other on exertional heat illness during training and competition (10), contain any reference to our definitive 1991 finding that fluid overload, and not sodium loss, is the preventable cause of EAH and EAHE (5).
Instead, a chorus of opinions from selected scientists was continuously recycled in the academic and lay press (11, 12) in order to counter our contrary but evidence-based findings. Both Position Stands invoke sodium deficits as factors in EAH and EAHE (9), and heat illnesses including muscle cramps (10)—a hypothesis for which, as I will show, there is no evidence. Both Position Stands promote the ingestion of sodium-containing drinks during exercise to prevent EAH and EAHE (9) or muscle cramping (10).
Ignoring the published evidence that conflicts with the selected material constitutes “cherry-picking,” an indefensible scientific crime, especially if that cherry-picked information may risk the safety of many athletes across the globe who, because they have no other option, place their faith in the pronouncements of the world’s most influential sports medicine and sports science organizations.
So, while the 2007 ACSM Position Stand on Exercise and Fluid Replacement (9) did finally acknowledge that overdrinking can cause EAHE, it also legitimized the theory that “salty sweaters” who lose substantial salt in their sweat and become both dehydrated and sodium-deficient during these races can also develop EAHE if they do not drink sodium-containing sports drinks at high rates. Subsequently, I will address this “dehydration-induced,” “salty sweater” EAH and EAHE mythology.
The clearest evidence that the industry chose to ignore our findings was in its publications (11, 12), which continued to explain, incorrectly, how EAH and EAHE are caused by high rates of sweat sodium losses during exercise.
Their explanation was simple and wrong. It proposed that EAH and EAHE are uncommon conditions that are caused by sodium deficiency in those “salty sweaters” who drink either too little or too much during more prolonged exercise. The condition can be exacerbated by water ingestion during exercise but is “minimized” by the ingestion of an electrolyte-containing (sodium-containing) sports drink.
According to this logic, it follows that the only way to remain healthy and optimize performance during prolonged exercise is to drink enough of a sodium-containing sports drink before, during, and after exercise to ensure that no weight is lost during exercise (the Zero-Percent Dehydration Doctrine). According to these experts, the widespread application of this approach would absolutely prevent EAH and EAHE.
The advice is especially attractive since it absolves everyone of any responsibility for the causation of EAH and EAHE without risking company profitability; it allows the industry to continue promoting the “drink as much as tolerable” dogma. Provided that the selected drink contains sodium, no one could be accused of promoting EAH and EAHE since they were only promoting what the scientists had themselves agreed by consensus to be the truth, as enshrined in the ACSM Position Stands (9, 10). The advice also maximizes product sales by encouraging athletes to drink more than they might actually need during exercise.
To construct this new myth, its proponents had to ignore a century of evidence showing that it is almost impossible to develop a salt/sodium deficiency in humans. And they, of course, had to ignore our evidence, published already in 1991, which showed that sodium deficiency is not present in EAH and EAHE (5).
Early Scientific Studies of Sodium Deficiency in Persons Exercising in the Heat
The search for sodium deficiency as a cause of ill health began with K. Neville Moss, Professor of Coal Mining at Birmingham University in the U.K. In 1923, he was the first to undertake studies of “heat” cramps (13).
Together with three iconic British physiologists — Professor J. S. Haldane, Professor Haldane’s son J. B. S. Haldane, and Professor A. V. Hill — Moss set out to study miners in Lancashire who developed muscle cramps during activity. Professor Haldane “suggested that cramp may depend upon excessive losses of chloride by sweating” (13, p. 196). As a result, an excited trio of Haldane, Jr., Hill, and Moss went to a “deep coal face at Pendleton Colliery and examined the [miners’] urine. A sample of urine obtained at the end of the shift from one of the colliers who was experiencing cramps was found to be practically free from chloride. This phenomenon, which is never present under normal conditions, and hardly ever at any time, made it quite clear that there was an excessive shortage of chloride in the blood (i.e., that the subject had developed a sodium deficiency, causing his muscle cramps)” (p. 196).
Moss’ solution was to provide miners with salt drinks made by the addition of 10 grams of sodium chloride per one gallon (3.79 liters) of water. This concoction had a sodium chloride concentration of 45 mmol/L (0.26%), which is approximately three times the concentration of Gatorade but still less than one-third the sodium concentration of blood (140 mmol/L). Four of seven miners treated with this solution felt they benefited, but one felt worse. “Curing” only four of seven subjects is not a meaningful effect.
Nevertheless, thus was born the theory that muscle cramping is due to a sodium deficit caused by excessive sodium losses in sweat — the forerunner of the Gatorade Sports Science Institute (GSSI)/ACSM “salty sweater” mythology — and compounded by excessive water intake.
An editorial entitled “Salt depletion by sweating,” published in September 1929 in the British Medical Journal, established this theory as fact: “Hard muscular work and the drinking of water to quench thirst were the two conditions which appeared to determine such attacks. It was clear that if a man was perspiring freely, and at the same time replacing the lost fluid by drinking water, there would be a tendency for the percentage of chloride in the blood plasma and in the whole body to fall (since much chloride might be lost in the sweat), with a corresponding tendency to a fall in the osmotic pressure of the blood plasma” (14).
This explanation sounds highly plausible but has to be wrong—for the reason that all creatures on Earth are designed to homeostatically regulate their blood sodium concentrations and ensure they remain constant within a tight range, regardless of how much salt or water is being lost or added to the body. The human model described in this editorial lacks any ability to survive, as it is unable to regulate its blood sodium concentration homeostatically.
This ideas in the editorial crossed the Atlantic, allowing Dr. John H. Talbot of the Harvard Fatigue Laboratory to write: “This lowering of the sodium and chloride in the serum, from loss in the sweat without adequate replacement is considered to be the principal causative mechanism in the production of heat cramps” (15, p. 359). He continues:
In the condition under discussion there is a loss of salt and water from the body with a replacement principally of water. If this major process is continued irrespective of the secondary processes, there will be a lowering of the sodium and chloride concentration below normal. When the critical level for sodium and chloride is reached in the working subject, muscle cramps will occur. (15, pp. 359-360)
As a result, prevention of the condition was relatively simple: “Since the cause of cramps is essentially a loss of salt in the sweat and urine, that is greater than the intake, the added ingestion of NaCl will prevent this negative balance” (15, p. 367).
Remarkably, more than 85 years later, in the 2007 ACSM Position Stands (9, 10) we find the evidence that the ACSM and its favored GSSI scientists rediscovered this explanation for both EAH and muscle cramping, even though it cannot be correct since:
(i) we have established that EAH is not caused by sodium deficiency;
(ii) EAH cannot occur solely as a result of overdrinking water. Rather there must also be abnormal fluid retention as a result of SIADH (or related phenomena);
(iii) this is because, as I have stressed, under all normal conditions, the blood sodium concentration is homeostatically regulated specifically to ensure that the loss of salt in sweat, or any other alteration, does not alter its concentration;
(iv) athletes who develop muscle cramps do not have low blood sodium concentrations (16-21), so a sodium deficiency cannot cause the condition; and
(v) persons with EAH and EAHE do not complain of muscle cramping, even though they can develop large increases in blood enzyme concentrations (22, 23) compatible with severely impaired muscle cell function.
In summary, the theory that muscle cramping is due to the development of a sodium deficit during exercise is based on the measurement of a single urine sample in a miner working at the Pendleton Colliery in Lancashire in the early 1920s. There is no other supportive evidence but much to disprove this theory. That it continues to be promoted by leading sports medicine organizations confirms the power of dogma, especially when it is underpinned by the financial muscle provided by commerce.
In Waterlogged (24), I present the much more probable explanation that muscle cramping has its origins in alterations in brain, skeletal muscle, and spinal cord function that are caused by exercise fatigue (18, 25).
Is It Possible to Produce a True Sodium Deficiency in Humans?
In the early 1930s, Dr. R. A. McCance, assistant physician in charge of biochemical research at King’s College Hospital in London, set out to determine if he could induce a state of sodium deficiency in healthy humans.
He realized that mammals that do not sweat can exist with very low sodium intake; thus, the most appropriate species for study would be sweaty humans losing sodium in their sweat. To produce a state of chronic sodium deficiency, he decided to expose a group of four subjects, two males and two females, to a very low-salt diet for a prolonged period while they were forced to sweat profusely, at least once a day.
To ensure that they ate a low-salt diet, Mrs. McCance kindly allowed the two male subjects to live at the McCance household with her husband while she fed them a salt-free diet comprising “salt-free ‘casein’ bread, synthetic sodium chloride-free milk, salt-free butter, thrice boiled vegetables, jam, fruit, home-made salt-free shortbread and coffee” (26, p. 823). To increase salt losses from the body, subjects lay in a radiant heat bath set at a temperature of 100°F (38°C) for two hours a day. This caused a sweat loss of between 2-3 liters. As a result, subjects lost about 1 kilogram of weight per day, or about 2-3 grams of sodium chloride.
Generally, it took “about a week” (equivalent to a sweat loss of 14-21 liters) to make the subjects “seriously deficient” (26, p. 823), by which time the total sodium chloride loss varied from 23 to 27 grams (390-460 mmol). McCance did not attempt to explain where all this sodium originated since it could not have come just from the extracellular space that comprises 15 liters and contains only 2,100 mmol. Had the sodium come only from the extracellular space, it would have lowered the blood sodium concentration by 22% to 110 mmol/L. Instead, blood sodium concentrations fell only to 137 mmol/L due to a large reduction in body weight (6-7 kg) and, I presume, movement of sodium into blood from the osmotically inactive exchangeable stores.
The subjects were then maintained in that state for three to four days. Common symptoms they developed included general lethargy and mild cramps, particularly of the muscles that were being used—for example, of the hands used for pipetting chemicals. McCance noted that the cramps were not of the severe, localized type previously described in miners but were “widespread, frequent, not very painful, and generally controllable” (27, p. 250).
During this time, urine sodium losses dropped steeply from ~3 g/day on day one to 0.3 g or less thereafter (28). By the end of the experiment, urinary sodium losses had fallen to 10 mg/day.
During recovery, subjects ate “highly salted foods such as anchovies and bacon” (26, p. 823). “We ate the bacon out of the frying pan to avoid losing any of its salt, and then, to make assurance doubly sure, we washed out the pan with a little hot water and drank the washings” (26, p. 823). To correct the original deficit, they needed to ingest the same amount of salt lost during the experiment (~20 g). Recovery was rapid as soon as >20 grams of salt had been ingested. This usually occurred overnight with a regain of all the lost weight.
Interestingly, the single subject who did not live with the McCance family failed to develop a sodium deficiency, and her data were not included (28). I suspect that cooking her own food proved too much of a temptation for the subject, who was unable to recreate Mrs. McCance’s salt-free cooking. Others have found it extremely difficult to generate a salt-deficiency in free-living humans.
Probably the most important conclusion of this study is that since we live in an environment in which salt is ubiquitous, cheap, freely available, and highly desirable, a sodium deficit is extremely difficult, perhaps impossible, to generate in free-living humans. Only under the most artificial laboratory conditions can such a deficit be produced.
Indeed, Y. Epstein and E. Sohar summarized what they had learned from 20 years studying sodium balance in persons exercising in desert heat in Israel: “A syndrome which is alleged to be due to pure salt deficiency as the name suggests … has never been proven to exist and is disproved by the findings cited above. Nor has sodium depletion due to salt loss in the sweat ever been observed in the extensive 20 years of field experience of the Environmental Unit at the Heller Institute at Tel-Hashomer” (29, p. 131).
As a result: “Intake of salt tablets during profuse sweating is not only unnecessary but may be harmful” (29, pp. 130-131).
These authors could be confident in their conclusions because they were consistent with the finding of others who, like McCance, had set out specifically to test the null hypothesis that it is (im-)possible to produce a sodium deficit in exercising humans.
And perhaps the reason is really quite simple: The osmotically inactive exchangeable sodium stores may be large and contain as much as 80% of the total body sodium stores. This intracellular store is filled progressively whenever sodium intake is high and then released gradually if sodium intake is reduced for any prolonged period.
Professor Jerome Conn, MD, Discovers the Hormonal Basis for Sodium Conservation by the Kidneys and Sweat Glands During Heat Acclimatization: An Unexpected Consequence of the Attack on Pearl Harbor
Dr. Jerome Conn, MD, was Professor of Internal Medicine at the University of Michigan, Ann Arbor, when the Japanese Air Force attacked the battleships of the United States Navy anchored at Pearl Harbor on Dec. 7, 1941.
The resulting entry of the U.S. into World War II soon occasioned a visit by members of the U.S. Armed Services to Professor Conn’s laboratory (30). They wished to know whether Conn would be prepared to study the processes of human acclimatization to heat. They were aware that in a war with Japan, their troops would be exposed to hot, humid conditions in the battle to capture the Pacific Islands before Japan could itself be attacked.
Because of his special expertise and interest, Conn decided to study sodium and water balance in conscientious objectors during an extended period of heat acclimatization. He began by studying subjects exercising in heat who lost 5 to 7 liters of sweat while ingesting different amounts of salt each day (30, 31).
As I have reviewed in great detail in Waterlogged (24), over the next decade or so, Conn made some pivotal discoveries that truly define our understanding of salt balance during exercise.
First, he showed that the sodium losses from the body always exactly matched the sodium intake, regardless of how little sodium the conscientious objectors were fed. In other words, like McCance’s studies, he found it extremely difficult, if not impossible, to produce a sodium deficit.
Second, he found that the body uses two mechanisms to reduce its sodium losses during exercise when daily sodium intake was reduced. The first was to reduce sodium losses in urine—this response happened almost immediately. The second was to reduce sodium losses in sweat. This mechanism was more delayed and usually took about 10 days to be maximized.
Third, he showed that urinary nitrogen excretion (due to increased protein breakdown) rose progressively when the daily salt intake was reduced. This reverted to normal levels some time after daily sweat salt losses had reached their lowest values.
Conn’s genius allowed him to conclude that all these effects must be due to the secretion of a hormone that (i) modifies sodium chloride losses in urine and sweat and (ii) increases protein breakdown. Since he knew that hormones secreted by the cortex of the adrenal gland produce both these effects, originally in his wonder, he termed the unknown substance “desoxycorticosterone-like adrenal steroid” (DLAS). In 1952, the hormone was isolated for the first time (32) and soon became renamed as aldosterone (33).
In subsequent studies, Conn measured changes in urinary and sweat sodium chloride excretion in response to progressive reductions in daily dietary salt intakes.
While there was a wide variation in these responses, most subjects could adapt to a daily salt intake as low as 6 g/day and some even lower, even when they exercised sufficiently to produce total sweat losses of up to 9 liters (34).
Some were able to achieve sodium balance even when ingesting only 1.9 g/day (31).
Conn concluded that the critical factors determining successful adaptation to heat were the speed at which and the extent to which the salt content of sweat losses could be reduced (30). Thus: “The metabolism of early acclimatization to heat was characterized by intense salt-saving activities of both the kidneys and the sweat glands” (30, p. 64). As a result, he wrote, “Probably the most sequential mechanism that we observed, was the ability of the acclimatized man to maneuver himself into a state of positive sodium balance” (30, p. 64). This would be a natural consequence of a mammal that evolved in a salt-deficient environment.
In his subsequent work, Conn sought to discover the nature of the hormone responsible for the coordination of all these changes. He concluded that the main determinant of successful acclimatization to heat was the ability to secrete a “salt-saving steroid” from the adrenal cortex. In time, it would become clear that the hormone involved in this mechanism is aldosterone.
Ultimately, Conn concluded that the process of heat acclimatization was the following: “The ability of the sweat glands to decrease the loss of salt from the skin to less than 5 per cent of the original loss, when necessary, makes it possible for the acclimatizing man receiving an average intake of sodium chloride to maneuver himself into a period of positive sodium balance with which he can now gradually increase his extracellular fluid volume” (30, p. 71).
“It thus seems reasonable to believe that one of the major functions of aldosterone is its indispensable place in bringing about the cutaneous, renal and cardiovascular adjustments which are necessary for man’s survival in hot climates,” he observed. It was therefore possible, he added, “to explain the results of acclimatization to heat almost wholly on the basis of increased production of aldosterone” (30, p. 72).
In April 1954, Conn was confronted with a patient with a complex disturbance in electrolyte metabolism (34). He concluded that all the abnormalities could be explained by the hypersecretion of aldosterone, termed primary hyperaldosteronism (35). It became apparent that the source was an aldosterone-secreting tumor of the adrenal cortex, an aldosteronoma. Surgical removal of the tumor cured the condition by causing a large urinary water and sodium excretion. Sweat sodium concentrations rose from 9 mmol/L to 45 mmol/L by the eighth postoperative day.
In honor of its discoverer, the condition is now known as Conn’s syndrome. The opposite condition is known as Addison’s disease. In Addison’s, a failure of adequate aldosterone secretion causes very high sweat sodium concentrations in excess of 60 mmol/L, which Conn considered to be the upper range of normal (36). Indeed, some patients with Addison’s disease can have sweat sodium concentrations as high as 110 mmol/L. This indeed is a cause of “salty sweating.”
Subsequently, Conn’s research team confirmed that aldosterone secretion increases in response to a period of heat acclimatization (37). They termed this condition secondary aldosteronism. They next showed that patients with primary hyperaldosteronism (Conn’s syndrome) may have a reduced capacity to transfer sodium into the exchangeable sodium stores, especially in the bone (38). When they then treated rats with aldosterone or DCA, there was a reduced uptake of sodium from blood to bone, whereas the aldosterone antagonist, spironolactone, increased sodium uptake by bone (39).
On the basis of these studies, they concluded that aldosterone may play a role “in regulating the transfer of electrolytes between bone and extracellular fluid” (38, p. 938). If correct, aldosterone would act to mobilize sodium from the osmotically inactive exchangeable sodium stores, increasing the blood sodium concentration. This effect of aldosterone could explain why 70% of athletes who gain weight during exercise do not inevitably develop EAH or EAHE (panels A and B in Figure 1). In contrast, the absence of this action would exacerbate the drop in blood sodium concentrations in those who overdrink (section C in Figure 1).
The point is that when the GSSI-favored scientists who drew up the 2007 ACSM Position Stands (9, 10) hinted that humans can become salt-deficient during exercise because of “salty sweating,” they had to ignore this remarkable body of work done by one of the most iconic U.S. medical scientists, as well as the reality that humans have a remarkable capacity to homeostatically regulate the blood sodium concentrations regardless of the imposed stresses.
Further Evidence That Sodium Deficiency Syndrome Simply Does not Exist
In Waterlogged (24), I presented all the evidence that a sodium deficiency simply does not exist in exercising humans. Here are two modern studies, the results of which are entirely predictable on the basis of what Conn discovered.
In 1975, David Costill and his colleagues showed that whether athletes ingested water or a prototypical electrolyte-containing “sports” drink, in this case Brake Time Thirst Quencher, during consecutive days of exercise in the heat, they remained in sodium (and potassium and chloride) balance for the duration of the experiment (40). As could be predicted from Conn’s studies, all the ingestion of the sports drink achieved was to increase (i) the total sodium ingested each day (Figure 2, top panel), and as a consequence, (ii) urinary sodium losses (Figure 2, bottom panel). Sweat sodium losses were unchanged (because the sodium challenge was too mild and the study too short).
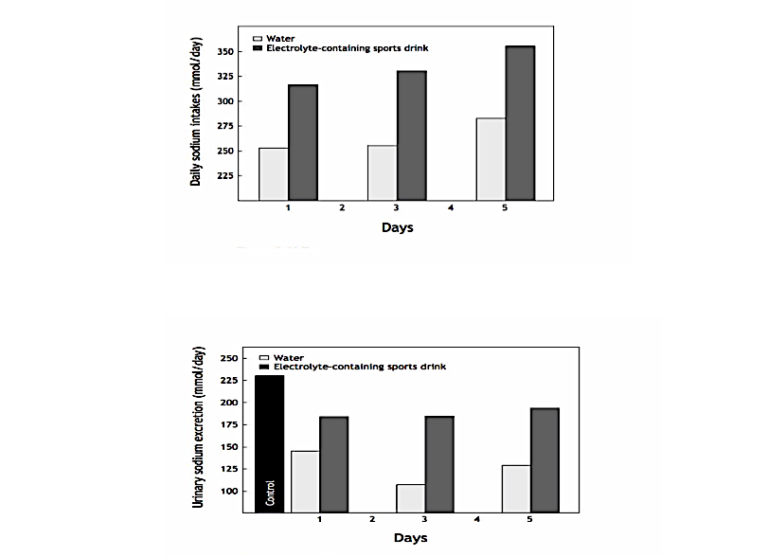
Figure 2, Top: Daily sodium intakes in subjects drinking either water or a sodium-containing sports drink during prolonged exercise in the heat. Bottom: Urinary sodium excretion on days one, three, and five of this experiment when either water or the sports drink was ingested. Note that all the extra sodium ingested in the sports drink appears in the urine and that daily urinary sodium losses are very high when the sports drink is ingested. The data indicate that subjects were habitually ingesting a diet with a very high sodium content: 225 mmol (~ 13 grams) per day. The sports drink added a further 25-75 mmol to this excess (Reproduced from reference 24, p. 141).
As a result, Costill’s group showed that the ingestion of Brake Time Thirst Quencher was an expensive method of increasing the amount of sodium excreted in the urine—a predictable response in athletes whose diets already contain a large sodium excess.
All these findings were reconfirmed in a study that exposed subjects to high temperatures for 10 hours a day for five days while they ingested one of three daily sodium intakes: 348 (20 grams), 174 (10 grams), or 66 (4 grams) mmol (41). Predictably, subjects maintained sodium balance by matching their urinary and sweat sodium losses exactly to their daily intakes (Figure 3, top panel). Daily urinary sodium losses were a function of dietary sodium intake and were reduced as a result of increased sweat sodium losses during the 10 days of heat exposure.
Yet, even with the lowest sodium intake (4 g/day), sodium was still present in the urine, indicating an absence of sodium deficiency. The sweat sodium losses (Figure 3, bottom panel) also fell during the period of heat exposure but were also a function of the dietary sodium intake. Excess sodium was still being lost in sweat, confirming the absence of a sodium deficiency.
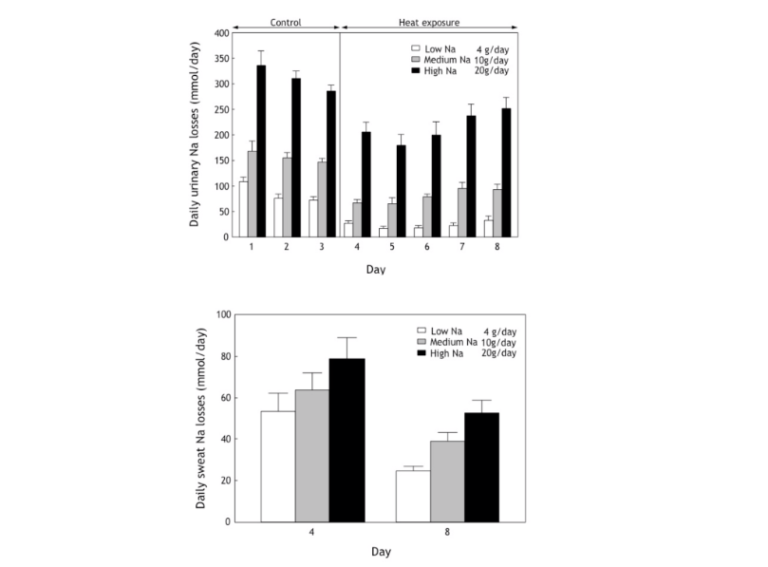
Figure 3, Top: Daily urinary sodium losses were reduced as a result of increased sweat losses during the 10 days of heat exposure (41). Yet, sodium was still present in the urine, even with the lowest sodium intake (4 g/day), indicating an absence of sodium deficiency. Bottom: The sweat sodium concentrations were also a function of the dietary sodium intake and fell during the period of heat exposure, as would be expected.
Numerous other studies, conveniently ignored by the GSSI and its scientists, have confirmed this biological law that humans regulate their total body sodium stores exquisitely accurately (42) despite any acute or chronic changes that may occur in the rates of either sodium intake or sodium losses (43-49).
Remarkably, some of these studies were conducted by Dr. Lawrence Armstrong, the senior author of the 2007 ACSM Position Stand on Exertional Heat Illness during Training and Competition (10). Included in that Position Stand is the following:
Prevention. Exercise-associated muscle cramps (EAMC) that occur in hot conditions seem to be prevented by maintaining fluid and salt balance. Athletes with high sweat sodium levels and sweat rates, or who have a history of Exercise-Associated Muscle Cramping, may need to consume supplemental sodium during prolonged activities to maintain salt balance (50-52, my reference notes) and may need to increase daily dietary salt to 5–10 g/day when sweat losses are large (53, 54, my reference notes). This is especially important during the heat acclimatization phase of training.
Predictably, four of the studies cited as evidence are reviews (50, 51, 53, 54), two of which were written by Armstrong and his friends (50, 51). The only study to include experimental data quantified the timecourse of body sodium losses in humans exercising in the heat (52). No attempt was made to show that such losses were detrimental or needed to be replaced as they developed.
Perhaps the final opinion belongs to F. Konikoff and colleagues, who concluded in 1986 that “acclimatized people living in a hot dry climate need no supplementary salt to their daily dietary intake while engaging in physical exercise or sport activities up to two hours a day. Salt loading under such conditions has no beneficial effects and, on the contrary, it may even be hazardous” (55, p. 300).
One wonders how this evidence was so easily ignored by those drawing up the 2007 ACSM Position Stands (9, 10).
Summary
Despite numerous serious attempts, only one study, that of McCance and colleagues, has been able to produce a state of acute sodium deficiency in healthy humans. This was only achieved by having subjects sweat profusely for up to two hours a day in the heat while eating a salt-free diet for more than a week. Free-living humans do not exist under such conditions.
Instead, easy access to added salt at meal times and humans’ natural proclivity to eat a salty diet ensures that free-living humans will not ever allow themselves to become salt-deficient.
