Dr. Robert Lustig, a pediatric neuroendocrinologist, has long been on the cutting edge of medicine and science. Throughout his forty-plus year career, The New York Times bestselling author of Fat Chance has been dedicated to treating and preventing childhood obesity and diabetes. With his new book, METABOLICAL: The Lure and the Lies of Processed Food, Nutrition, and Modern Medicine (Harper Wave; May 4, 2021; $28.99), he will expose the truth, both scientifically and politically, underlying the current global pandemic of diet-related diseases.
In his previous work, The Hacking of the American Mind, Dr. Lustig elucidated how the processed food industry has hacked our bodies and minds to pursue pleasure over happiness, fueling widespread addiction and depression. In METABOLICAL, he addresses nutrition, food science, and global health, and explains how by focusing on real food we can reverse chronic disease and promote longevity. For the first time, all strands of this pandemic — the medical, the economic, and the environmental — are pulled together into one clear narrative.
Describing the eight pathologies within the cell that belie all chronic disease, Dr. Lustig illustrates how they are not “druggable” but rather “foodable” (i.e. medication can’t cure what nutrition can) by following two basic principles: protect the liver, and feed the gut. He uses this science to chronicle the breakdown in our current healthcare paradigm, which has succumbed to influence from Big Food, Big Pharma, and Big Government. In the special chapter “Food in the Time of Corona,” Dr. Lustig addresses the way “pre-existing conditions” (i.e. diet-induced chronic diseases) make us vulnerable to succumbing to acute infectious diseases like COVID-19. He also argues that the Nutrition Facts label hides information from the consumer by omitting what’s been done to the food, which is more important than what’s in the food.
Weaving together the interconnected strands of nutrition, disease, medicine, environment, and society, METABOLICAL provides the scientific bases for a series of iconoclastic revelations, among them:
- Medicine for chronic disease only treats symptoms, not the disease itself
- You can diagnose your own biochemical profile
- Processed food isn’t just toxic, it’s addictive
- The war between vegan and keto is a false war—the combatants are on the same side
- Big Food, Big Pharma, and Big Government are on the other side
METABOLICAL will be available for purchase on May 4, 2021. Blurb and excerpt courtesy of Harper Wave.
Chapter 8. Checkpoints Alpha, Bravo, Charlie: nutrient-sensing and chronic disease
PART 2 (SEE PART 1)
Two metabolic pathways — one for growth, one for burning
Growing cells need all sorts of structural components in order to divide to make new cells. They need lipids for membranes, ribose (a 5-membered sugar) as the backbone for DNA and RNA, and amino acids for proteins. Where do all of these precursors come from? Imported via the blood? No, they are made on-site from available materials. Imagine a piece of wood in your house. That wood could be used to make a piece of furniture or it could instead be used for firewood, but not at the same time. Same for glucose inside the cell. Will it be used for growth and structural components or will it be burned? As we learned in chapter 7, there are two metabolic pathways inside the cell, that can either run in tandem (see Fig. 7-1). The first pathway is called glycolysis (see Chapter 7) — this process prepares glucose to be used for all these structural components for growth, which makes pyruvic acid as the goal as the end product of its metabolism. If it’s not used for burning in the next stage, it’s going to leave your cell as lactic acid. Glycolysis provides the added bonus of generating a grand total of 2 ATP’s, all without oxygen. The second pathway is called the Krebs Cycle — the pyruvic acid enters the mitochondria, where it burns it all the way to completion. You get the waste product carbon dioxide, but you also get the added bonus of liberating 28 ATP’s. If the goal is burning (e.g. aerobic exercise), you need both glycolysis and the Krebs Cycle. If the goal is only structural components for growth (why bodybuilders use High Intensity Impact Training to build muscle mass), then you only need glycolysis.
Nutrient sensing, kinases, and the set-up for chronic disease
Both of these two pathways, glycolysis for growth and the Krebs Cycle for burning, are adaptive. Basically, they’re the superhighways of your cell. If they get blocked, then the detours can be highly excruciating and kill you. When energy metabolism goes awry, those eight subcellular pathologies of Chapter 7 become maladaptive. How does processed food drive these pathologies? There are three protein checkpoints (like traffic lights) inside the cell called kinases that determine what happens to each molecule of glucose or fructose; these are turned on and off within seconds by the addition of a phosphate molecule generated from the food we eat. When the three checkpoints (which we’ll call Alpha, Bravo, Charlie) are coordinated in one direction, you get growth. When they are coordinated the opposite direction, you get burning. But when they are uncoordinated, that’s when we get a traffic jam and chronic metabolic disease happens.
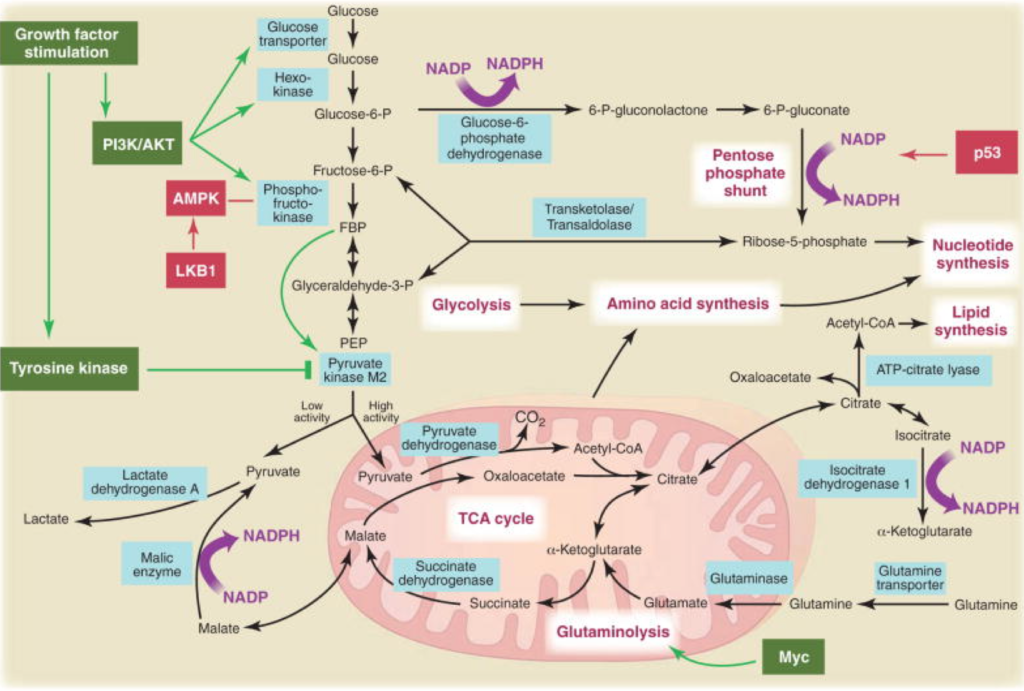
Fig. 8-1. The three enzymes that determine cell fate. PI3-kinase lets glucose into the cell; AMP-kinase directs that energy either to produce structural components for the cell or to enter the mitochondria to burn all the way to carbon dioxide; and mTOR determines whether the cell lives or dies.
Checkpoint Alpha: Phosphatidylinositol-3-kinase (PI3-kinase)
Glycolysis generates a total of 2 ATP’s from glucose molecule, hardly enough to power a cancer cell. But who said anything about just one glucose molecule? Cancer cells import 200 times the amount of glucose than normal cells do. They’re not making 2 ATP’s, they’re making 400. Lewis Cantley at Cornell showed that PI3-kinase opens the glucose floodgates (5). Cells have the fuel can produce enough energy to power themselves, all without mitochondria or oxygen.
So could blocking PI3-kinase stop cancer? Originally, drug trials of PI3-kinase inhibitors had been disappointing…until Cantley’s group showed that if you first cut down on insulin signaling of the cancer cell by reducing the amount of dietary refined carbohydrate, then the PI3-kinase inhibitors were much more effective (6). Insulin drives cancer cell growth because it is how the glucose gets into the cell in the first place; it’s the key to the door, and PI3-kinase determines how wide the door swings open (7).
Checkpoint Bravo: Adenosine monophosphate-kinase (AMP-kinase)
OK, the glucose gets in — then where does it go? If the cell is low on energy, it needs to burn. The second checkpoint, AMP-kinase, is the cell’s fuel gauge. It knows the difference between full and empty. When a cell has used up its ATP’s and the gas is empty, the mitochondria need to burn pyruvic acid completely to carbon dioxide, to generate 28 new ATP’s to replete the cell. AMP-kinase has the added bonus of signaling the cell to make more mitochondria in order to burn more glucose to make even more ATP. Anything that gooses AMP-kinase will keep intracellular fat down (8), and improve insulin sensitivity (9).
But when there is too much ATP, AMP-kinase gets turned off, mitochondria are not burning, and the cell will divert glucose to make structural components. Anything that impairs AMP-kinase will make fat, and worsen insulin resistance. What food impairs AMP-kinase the most? Sugar, of course (10; 11).
Checkpoint Charlie: Mammalian target of rapamycin (mTOR)
If a cell has plenty of energy but has limited oxygen or mitochondria, it may divide. If a cell has adequate oxygen and glucose, it may just hang out. Finally, if a cell has limited energy and is getting old, it may die to make room for new cells (autophagy, see Chapter 7). What signals this three-path Rubicon of cell fate? That’s the job of the third checkpoint called mTOR (12), which determines a cell’s commitment to growth, quiescence, or death. The discovery of mTOR highlights its central role in cell fate. In the late 1970’s, a soil sample from Rapa Nui (the native name for Easter Island) yielded a compound called rapamycin that had bizarre effects. Rapamycin turned to be not just an immunosuppressant, or an anti-cancer drug, or a fungicide — it’s all three, because it alters the growth phase of the cell (13). mTOR determines whether a cell lives or dies. It is the major regulator of mass accumulation in animals and is the key link between nutrient availability and the control of cell division versus cell death (14). mTOR is the “holy grail” of cell fate (15), and the target of most current “longevity drugs” (16). But because it is so multi-faceted, the medical establishment hasn’t yet figured out how to harness it.
As you might expect, mTOR is highly sensitive to diet (17). The protein composition of your diet activates mTOR, thereby promoting cell division, development of lean body mass, insulin sensitivity, and bone and cardiovascular health (18). Conversely, caloric deprivation (see Chapter 14) leads to lowering of ATP levels, which reduces mTOR all by itself. Activating AMP-Kinase can shut down mTOR in its tracks (19) (see Chapter 7). So while mTOR is its own checkpoint, it is also dependent on the cell’s AMP-kinase status. This will become important when we see how dyssynchrony of these three checkpoints can cause chronic disease.
- D.C. Wallace. “Mitochondria and Cancer: Warburg Addressed,” Cold Spring Harb. Symp. Quant. Biol. 70 (2005): 363.
- J. Lloyd-Price et al. ” The Healthy Human Microbiome,” Genome Med. 8 (2016): 51.
- R.H. Lustig et al. “Isocaloric Fructose Restriction and Metabolic Improvement in Children with Obesity and Metabolic Syndrome,” Obesity 24 (2016): 453.
- M.S. Conceição and C. Ugrinowitsch. “Exercise with Blood Flow Restriction: An Effective Alternative for the Non-Pharmaceutical Treatment for Muscle Wasting,” J. Cachexia Sarcopenia Muscle 10 (2) (2019): 257.
- R.J. Shaw and L.C. Cantley. “Decoding Key Nodes in the Metabolism of Cancer Cells: Sugar & Spice and All Things Nice,” F1000 Biol Rep 4 (2012): 2.
- B.D. Hopkins et al. “Suppression of Insulin Feedback Enhances the Efficacy of Pi3k Inhibitors,” Nature 560 (2018): 499.
- C. Greenhill. “Suppressing Insulin Feedback to Improve Efficacy of Cancer Therapeutics,” Nat. Rev. Endocrinol. 14 (9) (2018): 501.
- A. Woods et al. “Liver-Specific Activation of Ampk Prevents Steatosis on a High-Fructose Diet,” Cell Rep. 18 (13) (2017): 3043.
- B. Viollet et al. “Cellular and Molecular Mechanisms of Metformin: An Overview,” Clin. Sci. 122 (2012): 253.
- A. Gugliucci. “Fructose Surges Damage Hepatic Adenosyl-Monophosphate-Dependent Kinase and Lead to Increased Lipogenesis and Hepatic Insulin Resistance,” Med. Hypotheses 93: 87.
- A. Erkin-Cakmak et al. “Isocaloric Fructose Restriction Reduces Serum D-Lactate Concentration in Children with Obesity and Metabolic Syndrome,” J Clin Endocrinol Metab. 2019; 104 (7) (2019): 3003.
- R.A. Saxton and D.M. Sabatini. “Mtor Signaling in Growth, Metabolism, and Disease,” Cell 168 (2017): 960.
- J.B. Mannick and e. al. “Mtor Inhibition Improves Immune Function in the Elderly,” Science Transl. Med. 6 (2014): 268ra179.
- M. Perluigi et al. “Mtor Signaling in Aging and Neurodegeneration: At the Crossroad between Metabolism Dysfunction and Impairment of Autophagy,” Neurobiol. Dis. 84 (2015): 39.
- D.M. Sabatini. “Twenty-Five Years Obsessing over Mtor,” Proc Natl Acad Sci U S A. 114 (45) (2017): 11818.
- T. Weichhart. “Mtor as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review,” Gerontology 64 (2) (2018): 127.
- S. Sengupta et al. “Regulation of the Mtor Complex 1 Pathway by Nutrients, Growth Factors, and Stress,” Mol. Cell 40 (2010): 310.
- P. Nicklin et al. “Bidirectional Transport of Amino Acids Regulates Mtor and Autophagy,” Cell. 136 (2009): 521.
- Y. Li et al. “Tsc2: Filling the Gap in the Mtor Signaling Pathway,” Trends Biochem. Sci. 29 (2004): 32.
Comments on Metabolical: The Lure and the Lies of Processed Food, Nutrition, and Modern Medicine — Excerpt 2
mTOR is an interesting player embodying the struggle of growth versus longevity. Perhaps we need to decide how we wish to grow old and let that be the checkpoint for longevity?
Bought the Kindle version this morning. Thanks for yesterday’s email letting me know about the book’s release today. I’m looking forward to this, I’ve been a fan and student(follower) of Dr. Lustig since his 2010 “Sugar the Bitter Truth” lecture on Youtube. 👍👊

Metabolical: The Lure and the Lies of Processed Food, Nutrition, and Modern Medicine — Excerpt 2
2