The Origin of the Somatic Mutation Theory
For a brief historical perspective, let us consider how the somatic mutation theory (SMT) became the dominant explanation for the origin of cancer. The SMT and the view that cancer is a genetic disease originated with Theodor Boveri’s essay on the origin of malignant tumors, published in 1914 (19). Boveri had no prior research experience with cancer and based his views on the origin of tumors from observing abnormal cell division in sea urchins.
Indeed, Boveri states:
I have no personal experience worth mentioning in any of the numerous specialised fields of tumour research. My knowledge comes almost exclusively from books. Given this, it is inevitable that I am unaware of many reports in the literature, that I overestimate the significance of many known facts and that I do not set enough store by others. But this article will doubtless contain even more serious defects, as is so often the case when an author makes an incursion into a field with which he is unfamiliar.
In other words, the primary lens through which cancer’s clinical and research community views the disease today was developed by a person who, by his own admission, lacked a comprehensive understanding of the existing knowledge and practice related to cancer research at the time. (This should not, however, detract from Boveri’s monumental scientific achievement in linking Gregor Mendel’s inherited traits to chromosomes).
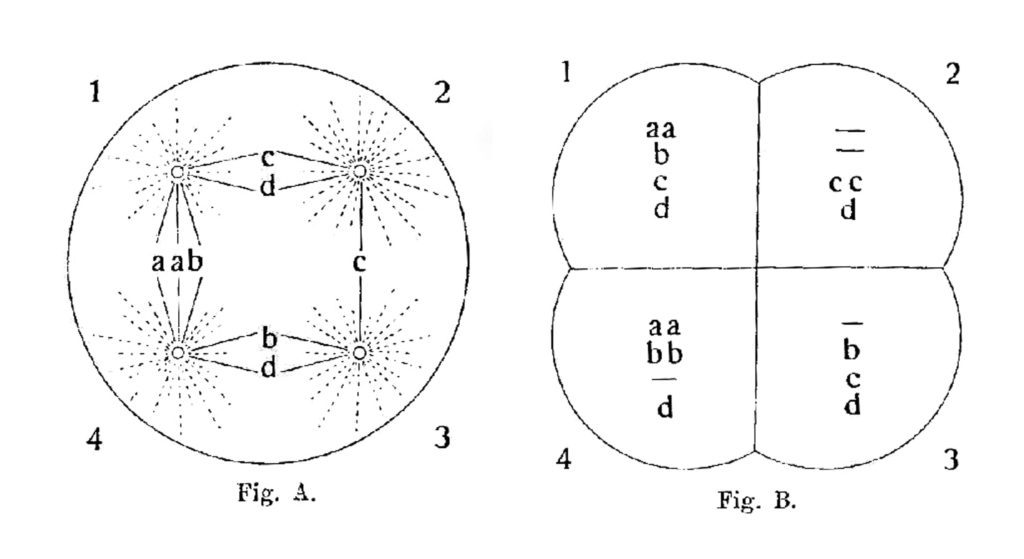
Boveri’s 1914 diagrams show a possible tetrapolar mitosis outcome for a cell containing eight chromosomes: 2a, 2b, 2c and 2d. Note that each daughter cell in this case receives half as many chromosomes as in bipolar mitosis, and most receive an uneven distribution of these chromosomes. (From 19).
Boveri proposed that all cancers arose in a single cell due to chromosomal imbalances or abnormalities. This “primordial tumorigenic cell” was a cell that:
Harbours a specific faulty assembly of chromosomes as a consequence of an abnormal event. This is the main cause of the propensity for unrestrained proliferation that the primordial cell passes to its progeny so long as these continue to multiply by normal mitotic binary fission. But all the other abnormal properties that the tumour cell exhibits are also determined by the abnormal chromosome constitution of the primordial cell, and these properties will also be inherited by all the progeny of this cell so long as subsequent cell division takes place by normal bipolar mitosis. (19)
Boveri stated categorically that “any theory of malignancy that does not take account of its unicellular origin is doomed” (19).
Despite Boveri’s inexperience, many of his conjectures regarding the origin of tumors have become tenets of the SMT, including the unicellular origin of cancer (6,20). A modern-day expansion of Boveri’s conjectures now includes a vast array of genetic mutations, including base-pair substitutions, frameshifts, deletions, duplications, and chromosomal translocations. Indeed, the degree of genetic heterogeneity found in tumors has exceeded all expectations, requiring unimaginable genetic classifications as drivers, passengers, gene hills and valleys, and even mysterious genetic dark matter (3). It is these types of observations that form the foundation of the biology of cancer and of the SMT (21).
Although the cell nucleus is the center of the SMT, Boveri could not exclude abnormalities in the cytoplasm as potentially responsible for the disease. He states:
Nevertheless, it has to be admitted that agents of a physical or chemical nature that act over longer periods might produce irreparable defects in the cytoplasm. And, indeed, certain specific facts that the study of tumours has revealed, such as the existence of cancers produced by X-rays or cancerous lesions simply called “chemical” carcinomas, make it seem probable that the hypothetical defects in the tumour cells in these cases do not belong to the category with which I have been solely concerned. (19)
In other words, Boveri acknowledges that cancer might also arise from defects in the cell cytoplasm. C. D. Darlington, the distinguished British geneticist, later emphasized the importance of the cytoplasm in the origin of cancer, rejecting the idea that cancer arises from genetic defects in the nucleus (22).
Darlington stated: “The development of unbalanced nuclei in tumours is without precedent in any living tissue. It implies a relaxation of detailed control in the nucleus which is also without precedent. And this in turn argues that the nucleus is not itself directly responsible for what is going on” (22).
However, many investigators have subsequently continued to view mitochondrial abnormalities as unimportant in the origin of cancer, but rather “simply another phenotype that is programmed by proliferation-inducing oncogenes” (2).
We will begin to consider science that challenges the SMT in Part 3 of our ongoing series exploring the origins of cancer.
Related
Thomas N. Seyfried is professor of biology at Boston College. He received a doctorate in genetics and biochemistry from the University of Illinois—Urbana-Champaign in 1976. He did his undergraduate work at the University of New England, where he recently received the distinguished Alumni Achievement Award. He also holds a master’s degree in genetics from Illinois State University. Seyfried served with distinction in the United States Army’s 1st Cavalry Division during the Vietnam War and received numerous medals and commendations.
He was a postdoctoral fellow in the Department of Neurology at the Yale University School of Medicine and then served on the faculty as an assistant professor in neurology. Seyfried previously served as chair of the Scientific Advisory Committee for the National Tay-Sachs and Allied Diseases Association. He recently received a Lifetime Achievement Award from the Academy of Complementary and Integrative Medicine and the Uncompromising Science Award from the American College of Nutrition for his work on cancer.
He presently serves on several editorial boards, including those for Nutrition & Metabolism, Neurochemical Research, the Journal of Lipid Research, and ASN Neuro. Seyfried has over 180 peer-reviewed publications and is author of the book “Cancer as a Metabolic Disease: On the Origin, Management, and Prevention of Cancer” (Wiley Press).
References
Note: These references include those previously published in “Is Cancer a Genetic or Metabolic Disease? Part 1.”
- Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68.1(2018): 7-30. Available here.
- Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell 144.5(2011): 646-674. Available here.
- Vogelstein B, Papadopoulos N, Velculescu VE et al. Cancer genome landscapes. Science 339.6127(2013): 1546-1558. Available here.
- Hou JP and Ma J. DawnRank: discovering personalized driver genes in cancer. Genome Medicine 6.7(2014): 56. Available here.
- Iranzo J, Martincorena I, and Koonin EV. Cancer-mutation network and the number and specificity of driver mutations. Proceedings of the National Academy of Sciences of the United States of America 115.26(2018): E6010-E6019. Available here.
- Fearon ER and Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 61.5(1990): 759-767. Available here.
- Tomasetti C and Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347.6217(2015): 78-81. Available here.
- Vaux DL. In defense of the somatic mutation theory of cancer. BioEssays 33.5(2011): 341-343. Available here.
- McLeod HL. Cancer pharmacogenomics: early promise, but concerted effort needed. Science 339.6127(2013): 1563-1566. Available here.
- Ju J, Zhu A, and Yuan P. Progress in targeted therapy for breast cancer. Chronic Diseases and Translational Medicine 4.3(2018): 164-175. Available here.
- Seyfried TN. Cancer as a Metabolic Disease: On the Origin, Management and Prevention of Cancer. Hoboken, New Jersey: John Wiley & Sons, Inc., 2012. Available here.
- John AP. Dysfunctional mitochondria, not oxygen insufficiency, cause cancer cells to produce inordinate amounts of lactic acid: the impact of this on the treatment of cancer. Medical Hypotheses 57.4(2001): 429-431. Available here.
- Kim A. Mitochondria in cancer energy metabolism: culprits or bystanders? Toxicological Research 31.4(2015): 323-330. Available here.
- Pelicano H, Zhang W, Liu J et al. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: role of mTOR pathway and therapeutic potential. Breast Cancer Research 16.5(2014): 434. Available here.
- Srinivasan S, Guha M, Dong DW et al. Disruption of cytochrome c oxidase function induces the Warburg effect and metabolic reprogramming. Oncogene 35.12(2016): 1585-1595. Available here.
- Stefano GB and Kream RM. Cancer: mitochondrial origins. Medical Science Monitor 21(2015): 3736-3739. Available here.
- Warburg O. On the origin of cancer cells. Science 123.3191(1956): 309-314. Available here.
- Chinopoulos C and Seyfried TN. Mitochondrial substrate-level phosphorylation as energy source for glioblastoma: review and hypothesis. ASN Neuro 10(2018): 1-27. Available here.
- Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. Journal of Cell Science 121. Suppl.1(2008): 1-84. Available here.
- Nowell PC. The clonal evolution of tumor cell populations. Science 194.4260(1976): 23-28. Available here.
- Weinberg RA. The Biology of Cancer. New York City, New York: Garland Science, 2007. Available here.
- Darlington CD. The plasmagene theory of the origin of cancer. British Journal of Cancer 2.2(1948): 118-126. Available here.
All links accessed Jan. 24, 2019.
Comments on Is Cancer a Genetic or Metabolic Disease? Part 2
I like how Boveri himself already had some parts of the truth. It just didn’t catch the attention of the scientist at the time? Or is there a reason why no one at the time challenged is position?

Is Cancer a Genetic or Metabolic Disease? Part 2