Question: Diabetes and obesity have been shown to increase risk of asthma. Independent of the effect of these conditions on inflammation, what mechanisms might mediate this risk increase?
Takeaway: Insulin can lead to changes in muscular and epithelial cells in the lungs as well as changes in the extracellular matrix — all of which may contribute to asthma and similar conditions.
Extensive epidemiological evidence indicates obesity, metabolic syndrome, and insulin resistance increase asthma risk (1) and in particular suggests the relationship is driven by metabolic dysfunction (i.e., hyperglycemia, hyperinsulinemia, and/or insulin resistance), not by obesity. Multiple observational studies have directly observed that markers of metabolic distress are associated with asthma risk, independent of body weight (2).
This brief 2013 review summarizes mechanisms by which poor metabolic health may increase asthma risk. Notably, it does not consider any relationship between inflammation and asthma risk; given the potential contribution of inflammation to asthma incidence and severity, pro-inflammatory metabolic distress may exacerbate asthma beyond the factors discussed here (3).
Lung cells are responsive to insulin (4). Some evidence suggests excessive insulin exposure can impair lung function. Inhaled insulin leads to reduced expiratory volume in diabetics (5), and inhaled insulin was withdrawn from the market due to reports of persistent respiratory problems (6).
Airway hyperresponsiveness (AHR) — that is, excessive narrowing of the airway — is a primary feature of asthma (7). Insulin may lead to AHR through its effect on various lung tissues (8). Multiple animal studies have shown increased insulin levels induce proliferation of airway smooth muscle (ASM) cells and promote ASM contraction. Increased insulin exposure leads to cellular remodeling, changes to the extracellular matrix (ECM), and thicker, stiffer hypercontractile airways (9). Many of these proposed mechanisms are driven by the effect of insulin on PI3K, a major regulator of cellular growth and proliferation.
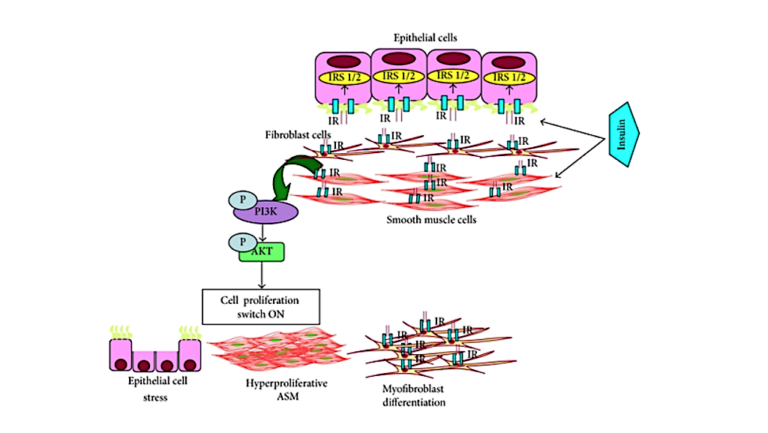
Figure 1: A proposed model by which increased insulin can lead to lung cell remodeling and asthma pathology
Most of these data are drawn from animal studies, in vivo research, or inference from other similar conditions; as such, it is premature to conclude there is a direct link between hyperinsulinemia and asthma. Instead, these mechanisms have the potential to explain the observed relationship between poor metabolic health and increased asthma risk. They also indicate potential therapies to test for the prevention and/or treatment of asthma.
Notes
- Update on asthma control in five European countries: Results of a 2008 survey; Burden of comorbidity in individuals with asthma; Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies; Insulin resistance as a predictor of incident asthma-like symptoms in adults; Understanding asthma and the metabolic syndrome — A Nigerian report; Metabolic syndrome and incidence of asthma in adults: The HUNT study; Diseases concomitant with asthma in middle-aged and elderly subjects in Korea: A population-based study
- Association of obesity and insulin resistance with asthma and aeroallergen sensitization; Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease; The metabolic syndrome; Is prevalence of metabolic syndrome high in patients with asthma?; Systemic inflammation and metabolic syndrome in stable COPD patients
- The relationship of inflammatory cytokines with asthma and obesity; Obesity and asthma; Cluster analysis and clinical asthma phenotypes
- A comparison of insulin receptors in the developing fetal lung in normal and in streptozotocin-induced diabetic pregnancies; Pulmonary insulin responsivity: in vivo effects of insulin on the diabetic rat lung and specific insulin binding to lung receptors in normal rat; Plasma membrane insulin receptors in fetal rabbit lung
- Meta-analysis: Efficacy and safety of inhaled insulin therapy in adults with diabetes mellitus; Inhaled insulin for diabetes mellitus
- NIDDM patients’ fears and hopes about insulin therapy: The basis of patient reluctance; Inhaled insulin is approved in Europe and United States
- Biphasic late airway hyperresponsiveness in a murine model of asthma
- Leukotriene D4 facilitates airway smooth muscle cell proliferation via modulation of the IGF axis
- Insulin induces a hypercontractile airway smooth muscle phenotype; Airway smooth muscle in asthma: Phenotype plasticity and function; Role of Rho-kinase in maintaining airway smooth muscle contractile phenotype; Insulin causes a transient induction of proliferation via activation of the PI3-kinase pathway in airway smooth muscle cells; Insulin increases the expression of contractile phenotypic markers in airway smooth muscle; Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype; Laminin isoforms in tumor invasion, angiogenesis and metastasis; Insulin induces airway smooth muscle contraction
Comments on Insulin and the Lung: Connecting Asthma and Metabolic Syndrome
Résumé/traduction rapide
Insuline et les poumons : Connecter l’asthme et le syndrome métabolique
Question abordée : Les diabètes et l’obésité augmentent le risque d’asthme. Indépendamment des effets de ces maladies sur l’inflammation, quels mécanismes peut diminuer cette augmentation de risque ?
Conclusion rapide : L’insuline peut provoquer des changements au niveau des cellules musculaires et épithéliales dans les poumons ainsi que des changements dans la matrice extra cellulaire. Ces cellules peuvent toutes contribuer à l’asthme et à d’autres conditions.
___
Des preuves épidémiologiques indiquent que l’obésité, le syndrome métabolique et la résistance à l’insuline augmentent le risque d’asthme et en particulier suggère que la relation est dirigé par le dysfonctionnement métabolique (hyperglycémie, hyperinsulinémie et/ou résistance à l’insuline) et pas par l’obésité.
De nombreuses études montrent que les marqueurs de détresse métabolique sont associés avec le risque d’asthme indépendamment du poids du corps.
Les cellules du poumon sont sensibles à l’insuline. Des preuves montrent que l’exposition à l’insuline peut diminuer la fonction du poumon.
L’insuline peut aussi mener à une réduction des voix respiratoires à travers ces effets sur des tissues pulmonaires.
Il est encore prématuré de lier l’hyperinsulinémie et l’asthme.
Mais ces mécanismes peuvent potentiellement expliquer le lien direct entre une mauvaise santé métabolique et l’augmentation du risque de l’asthme.
Elles indiquent également des thérapies potentielles pour tester/traiter l’asthme
Merci beaucoup pour la traduction Julian ! Encore une grande aide pour tous les CrossFiters de France.
Insulin and the Lung: Connecting Asthma and Metabolic Syndrome
2