This review summarizes evidence and describes the mechanisms that suggest fructose specifically induces visceral adiposity through its metabolic impact, not merely its caloric value. Excessive fructose consumption leads to individuals termed TOFI, “thin on the outside, fat on the inside,” and thus individuals who are more metabolically unhealthy than their weight or BMI would indicate.
Authors James J. DiNicolantonio, Varshil Mehta, Neema Onkaramurthy, and James O’Keefe write:
Traditionally, the leading hypothesis regarding the development of obesity involves caloric imbalance, whereby the amount of calories consumed exceeds the amount of calories burned which causes obesity. Another hypothesis for why we get fat has surfaced in the last decade, which is the idea that the overconsumption of added sugars and refined carbohydrates induce insulin resistance and high insulin levels causing obesity. While insulin is a fat-storing hormone, this hypothesis does not explain visceral adiposity, or why certain people are found to have fat stored in and around their organs. We [James J. DiNicolantonio et al.] propose a new mechanism for body fattening, particularly visceral adiposity. This hypothesis involves the overconsumption of fructose, which leads to inflammation in all cells that metabolize it rapidly.
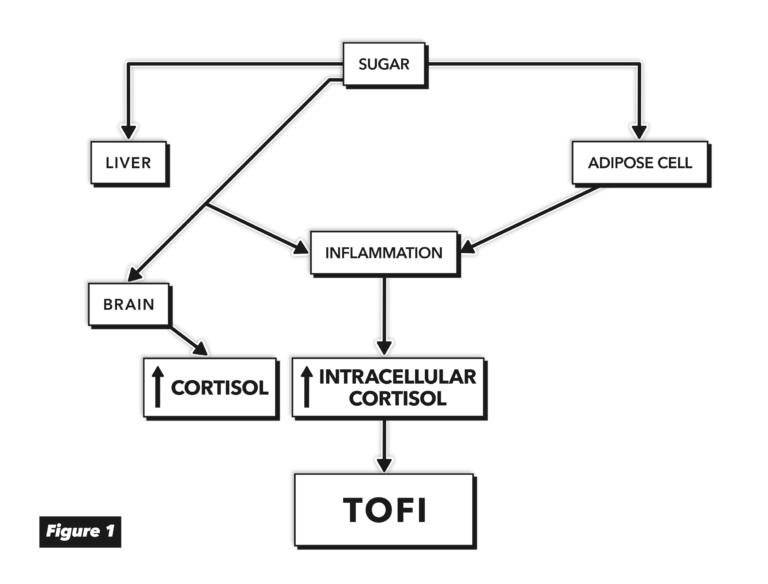
Figure 1, adapted from Fructose-Induced Inflammation and Increased Cortisol: A New Mechanism for How Sugar Induces Visceral Adiposity
Fructose can be absorbed by a variety of tissues, including adipocytes, the kidneys, muscles, and brain. Fructose consumption, the authors argue, directly causes inflammation in the adipose tissue through multiple mechanisms, in some cases triggering vicious cycles via its impact on leptin, reactive oxygen species, and increased cellular cortisol levels. When the adipose tissue becomes insulin resistant, the flux of fatty acids out of the subcutaneous adipocytes increases. These fatty acids are deposited in the liver, which also has upregulated fatty acid production due to the intake of sucrose. Normally, these absorbed and produced fatty acids are secreted as very low-density lipoprotein (VLDL) and released into the blood. However, when the flux of fatty acids into the liver exceeds what VLDL production can remove, fat begins to deposit in the liver itself. This leads to hepatic insulin resistance and further increases VLDL production and, eventually, liver inflammation.
These and other mechanisms simultaneously drive the release of free fatty acids from subcutaneous adipocytes and their storage in the liver and other visceral tissues. Each mechanism is explored in detail in this paper. These effects, which in some cases negatively impact organ function, are all independent of any impact fructose has on overall adiposity or weight.
In summary, the authors argue fructose consumption directly triggers pathways leading to insulin resistance (especially in the liver and fat tissue), inflammation, and increased cortisol levels, which collectively make fructose specifically harmful in ways other carbohydrates are not.
Comments on Fructose-Induced Inflammation and Increased Cortisol: A New Mechanism for How Sugar Induces Visceral Adiposity
Added fructose is a tried and true method researchers use to create insulin resistance in lab animals for study. And though mice and rats are not just furry little humans, there are human parallels.
Obviously table sugar is half fructose, with the sucrose disaccharide quickly split into its glucose and fructose halves by the digestive apparatus. But HFCS can be as little as 42% fructose all the way up to 55% fructose. So it can have less or more fructose than table sugar. The moniker 'high fructose' derives from the fact that pure 'corn syrup' is 100% glucose.
Although there is fructose (and glucose) in fruits, it's not the fiber so much as the absolute quantity of fructose that makes eating fruit less problematic than concentrates. For instance, that banana has about 7 grams of fructose in it, compared to about 20 in a standard can of HFCS sweetened soda. A cup of strawberries has just 4 grams of fructose; by comparison 5x less than a soda. Most people aren't going to eat 3 or 4 bananas or 5 cups of strawberries at a sitting, but many of them can throw down the 32 oz Big Gulp, which is nearly 3 standard 12 oz cans of soda, containing almost 60 grams of fructose. And they might refill it!
The effects of fructose, metabolically, when consumed in quantity, are devastating because there is no good metabolic governor on it, like there is for glucose. It sort of jumps the control point gap, making it even more insidious and dangerous than equivalent amounts of concentrated glucose. Suffice it to say that they're all --table sugar, corn syrup, HFCS alike -- concentrated, readily-absorbed sugars in the end and have no place as a regular part of a healthy human diet. And I would add to that list all the so-called paleo sugars, such as coconut sugar (35% to 40% fructose and the rest glucose) and maple syrup which tends to be more glucose than fructose by about 2:1 or more, depending on grade. Sugar is sugar and needs to be eaten sparingly and mainly by being extracted by the human gut from the whole foods it sparingly occurs in.
So does this pertain to artificially added fructose in foods or whole fruits (bananas etc) that are consumed with their natural fiber. Please advise! Thanks
It is in regards to excessive quantities (unsure what level) of something like High Fructose Corn Syrup (refined) which is easily overingested in processed foods. In theory eating excessive amount of fruit over a long period of time could cause the same effect, but the actual quantity of fruit would be excessive in itself.
This is dangerous summarising on CrossFits behalf. The article is a literature review pertaining to added fructose, as opposed to fruit itself. This needs to be adapted in CrossFit’s summary of the article. As you can see the first comment is unsure as to what the article relates to. The article itself in two places states this does not relate to fruit and that fruit consumption has health benefits.
Hey Ashutosh, great question.
I think there are two elements to that answer. One Andrew already addressed - simply, the volume of sugars present in natural foods is, in the vast majority of cases, much less than that seen in artificially sweetened foods. (As a quick comparison, one banana, a relatively sugary fruit, has 14g of sugar, compared to 39g in a 12oz Coke) But second, the data we do have suggests that, whatever the mechanism, fructose in fruit does not seem to be associated with metabolic harm. (The authors briefly address this twice in the paper) I've read some authors suggest this is due to fiber suppressing the fructose response, and others have argued that the beneficial nutrients in fruit offset the inflammatory and oxidative impact of fructose.
I'm not clear on the precise mechanism, but I do know that fatty liver disease clinicians I've spoken with seem to consistently focus on removing added sugar specifically from the diet (and potentially concentrated natural sugars, like some fruit juices), as this removes the majority of the sugar and so alleviates the majority of any harm fructose may be causing.
Great question. I agree with Clarke and Dr Eades. No kid will eat 3 apples. It takes too long, they don’t sit down long enough. And they are full. And sick of chewing. But kids can quickly pound a glass (or two) of apple juice (or other sweetened beverages). SSBs are like giving an IV bolus of sugar.
Thanks all for answering. It seems once again like eating real food is the best and staying away from process sugar and over transformed carbohydrates.
Fructose-Induced Inflammation and Increased Cortisol: A New Mechanism for How Sugar Induces Visceral Adiposity
7