This comprehensive 2019 New England Journal of Medicine review summarizes the impact of intermittent fasting at both a cellular and an organ/body system level. It then outlines how these changes may benefit individuals with a variety of disease states.
Intermittent fasting, as defined by review authors Rafael de Cabo and Mark P. Mattson, is a period of food restriction sufficient to clear liver glycogen stores and dramatically suppress glucose, insulin, and amino acid uptake by cells. During fasting, fatty acids are released into circulation and taken up by liver cells, which produce ketone bodies to be used as fuel by other cells. These changes result in a cascade of systemic and cellular responses related to the suppression of insulin and IGF, energy depletion in cells, and the regulatory impact of ketone bodies (1). Diets that induce this change include complete abstention from food for one or more days (as is done in some forms of alternate-day fasting and 5:2 fasting), as well as time-restricted feeding (where food is only eaten during a limited number of hours each day) and near-fasting regimens (where daily caloric intake is restricted to 500-700 calories (2).
Intermittent fasting downregulates pathways related to cellular metabolism, IGF-1, insulin, and amino acid signaling. These changes activate cellular repair, maintenance processes, stress resistance, and mitochondrial biogenesis, which in turn support repair of cellular damage and cell survival; subsequent refeeding upregulates biogenesis and growth (Figure 1).
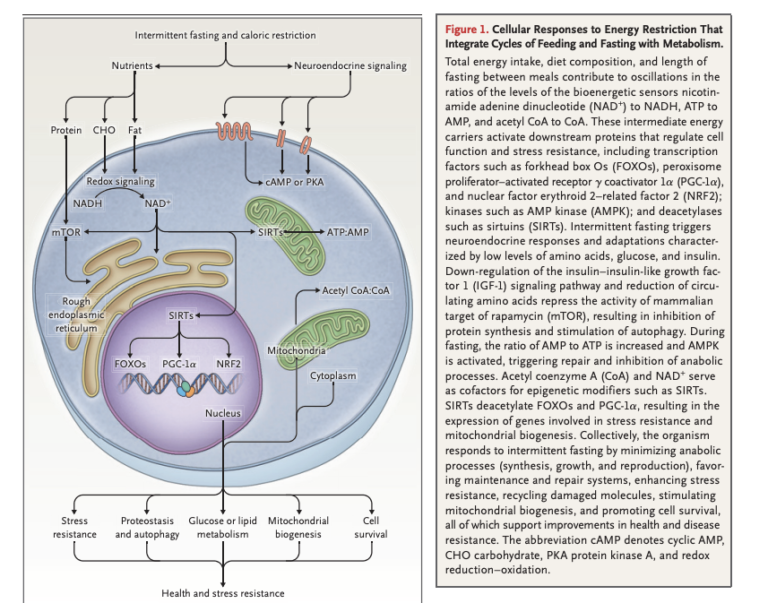
After 10 to 14 hours, liver glycogen stores are depleted, and free fatty acids (FFAs) are released into circulation from the adipose tissue. The liver converts these FFAs into ketone bodies, which provide fuel for other tissues. Systemically, this leads to a variety of changes across multiple organ systems related to reduced cellular energy availability and reduced activation of pathways related to glucose and insulin (Figure 2).
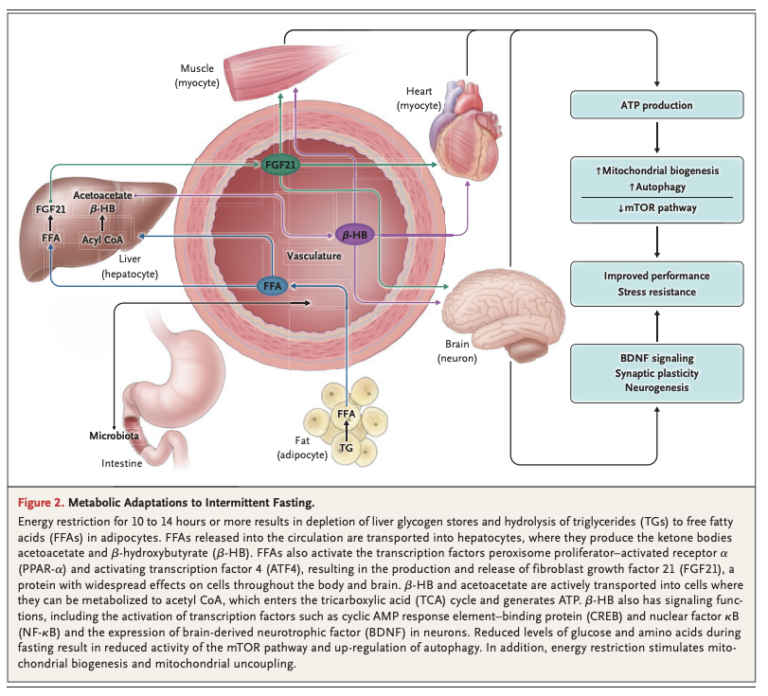
These metabolic responses are similar across a variety of organisms, suggesting they are evolutionarily conserved. Note individuals eating a typical American diet, with three daily meals and snacks in between, will rarely, if ever, reach a fasting state (3).
Animal research has consistently shown that caloric restriction, whether chronically or through intermittent fasting, improves lifespan (4). This same data suggests sex, age of diet initiation, and the composition of the remaining calories can have a significant impact on the degree of benefit from caloric restriction; some regimens show greater benefit than others, but these differences lack sufficient consistency to clearly indicate the determining factors. Human studies including the Okinawans and members of the Caloric Restriction Society suggest long-term compliance with fasting is associated with longevity and low levels of metabolic disease (5).
Figure 3, below, outlines the potential impact of fasting regimens on specific markers of disease.
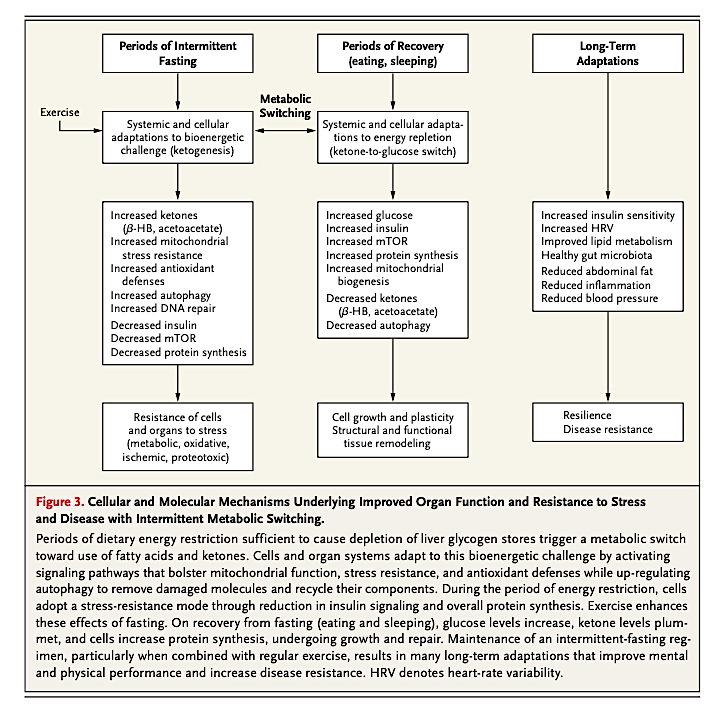
Periods of intermittent fasting increase cellular stress resistance and defense. They also repair pathways, thereby improving the resistance of cells to a variety of stressors. Periods of recovery (i.e., post-fast refeeding) increase cellular synthesis and growth. Regular “metabolic switching” between these two states promotes long-term maintenance of a variety of organ systems, disease resistance, and improved performance.
Human trials suggest intermittent fasting confers greater improvements with regard to insulin sensitivity and fat mass in overweight and/or diabetic subjects than similar levels of caloric restriction or other diets leading to similar weight loss (6).
Human and animal trials suggest intermittent fasting improves cognitive function, specifically various forms of memory. This means fasting may prevent or slow the progression of cognitive disease — particularly interesting given the lack of viable alternative treatments (7). These benefits may be related to improvements in neuronal mitochondrial function, antioxidant defenses, and/or DNA repair (8).
Human studies have also shown intermittent fasting improves markers of cardiovascular disease risk, including blood pressure, heart rate, lipid levels, and markers of inflammation and oxidative stress (9).
Animal studies have shown intermittent fasting reduces spontaneous tumor occurrence. It also suppresses the growth of a variety of tumors while increasing the susceptibility of tumor cells and the resistance of healthy cells to chemo and radiation (10). Similar human studies are in the early stages, but preliminary case studies and trials suggest the vast majority (~95%) of cancer patients can adhere to a fasting regimen without side effects, and for some types of cancers, fasting may suppress tumor growth and increase survival (11).
Preliminary evidence similarly indicates intermittent fasting may be beneficial in patients with inflammatory and autoimmune conditions, such as asthma, MS, and arthritis; animal and human studies have demonstrated functional improvements in as little as two months when patients with MS begin a fasting regimen (12).
Finally, intermittent fasting has been found to improve outcomes and reduce tissue damage related to surgery and traumatic injury, including traumatic brain and/or spinal cord injury (13).
The review studies indicate initiating fasting is challenging but feasible, particularly if patients are prepared for the major barriers — namely, the ingrained culture of eating three meals per day and the temporary hunger, irritability, and poor concentration patients experience during the first month following a fasting regimen.
Takeaway: The majority of fasting research remains limited to animal research and must be treated with caution with regard to human populations. However, the existing evidence and our mechanistic understanding of the impact of fasting consistently suggest the simultaneous suppression of glucose and insulin levels and upregulation of ketones induced by fasting lead to stress resistance, cellular repair, mitochondrial biogenesis, and upregulation of a variety of cellular maintenance processes, all of which may lead to improvements in a wide variety of conditions linked to poor metabolic health.
Notes
- Caloric intake and aging; Circadian physiology of metabolism: A time to fast; Fasting: Molecular mechanisms and clinical applications; Intermittent metabolic switching, neuroplasticity and brain health; Caloric restriction improves health and survival of rhesus monkeys; Caloric restriction and cancer: Molecular mechanisms and clinical implications; Fasting and cancer: Molecular mechanisms and clinical application; Caloric restriction (Speakman JR); Flipping the metabolic switch: Understanding and applying the health benefits of fasting; Hallmarks of brain aging: Adaptive and pathological modification by metabolic states
- Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma; Hypocaloric diets and ketogenesis in the management of obese gestational diabetic women; Short-term very low calorie diet reduces oxidative stress in obese type 2 diabetic patients; The effects of intermittent or continuous energy restriuction on weight loss nad metabolic disease risk markers: A randomized trial in young overweight women
- An evolutionary perspective on why food overconsumption impairs cognition
- Dietary restriction in rats and mice: A meta-analysis and review of the evidence for genome-dependent effects on lifespan; Fat maintenance is a predictor of the murine lifespan response to dietary restriction; Genetic variation in the murine lifespan response to dietary restriction: From life extension to life shortening; Caloric restriction delays disease onset and mortality in rhesus monkeys; Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study
- Caloric restriction and human longevity: What can we learn from the Okinawans?; Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans; Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding protein and cortisol in nonobese men and women: A randomized clinical trial; Calorie restriction in humans: An update
- The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young, overweight women; The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women; Alternate-day fasting in nonobese subjects: Effects on body weight, body composition and energy metabolism; Design and conduct of the CALERIE study: Comprehensive assessment of the long-term effects of reducing intake of energy; Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance; etc.
- Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges; Effects of weight weeks of time-restricted feeding (16/8) on basal metabolism, maximial strength, body composition, inflammation and cardiovascular risk factors in resistance-trained males; Cognitive and behavioral evaluation of nutritional interventions in rodent models of brain aging and dementia; Caloric restriction improves memory in elderly humans; Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment; The effect of caloric restriction on working memory in healthy non-obese adults
- Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities
- Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats; Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans; A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity; Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals; Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations
- When less may be more: Calorie restriction and response to cancer therapy; Energy restriction and the prevention of breast cancer; Calories, carbohydrates and cancer therapy with radiation: Exploiting the five R’s through dietary manipulation; Cancer metabolism: A therapeutic perspective; Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction
- Feasibility outcomes of a presurgical randomized controlled trial exploring the impact of caloric restriction and increased physical activity versus a wait-list control on tumor characteristics and circulating biomarkers in men electing prostatectomy for prostate cancer; Management of glioblastoma multiforme in a patient treated with ketogenic metabolic therapy and modified standard of care: A 24-month follow-up
- Diet-induced weight loss in obese children with asthma: A randomized controlled trial; A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms; Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota; Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis; Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review
- Reducing elective vascular surgery perioperative risk with brief preoperative dietary restriction; Preoperative dietary restriction reduces intimal hyperplasia and protects from ischemia reperfusion injury; Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: A randomized multicenter study; Short term caloric restriction exerts neuroprotective effects following mild traumatic brain injury by promoting autophagy and inhibiting astrocyte activation
Effects of Intermittent Fasting on Health, Aging, and Disease