These two 2012 reviews — one by Barbara Corkey, Ph.D., and the other by Walter Pories, MD and FACS, and G. Lynis Dohm, Ph.D. — both argue the root cause of diabetes is excessive levels of insulin, and insulin resistance is a secondary rather than primary cause.
Since the 1960s, the prevalence of diabetes in the U.S. population has increased from ~5% to more than 15% (1). Current standard-of-care treatments include diet and exercise to reduce weight and use of oral hypoglycemic medications like metformin or injected (exogenous) insulin to reduce blood glucose levels. These treatments are unable to reverse the condition; for nearly all diabetics, diabetes is a lifelong condition that worsens over time, leading to the deterioration of cardiovascular, neurological, optical, and kidney function (2).
As Corkey notes, “These realities indicate that both our understanding of the disease and our treatment of the disease are inadequate. Current approaches are not working” (2432).
The current standard of care, as well as the general clinical and academic perception of the disease, assumes the primary cause of diabetes and its progression is insulin resistance. In brief, the muscles, fat tissue, and liver become insulin resistant; as muscles and fat tissue become insulin resistant, blood glucose disposal decreases, and as the liver does, blood glucose production increases. Combined, these two changes lead to chronically elevated blood glucose levels. Chronically elevated insulin levels, in this framework, are merely the body’s attempt to reestablish normal blood glucose levels as the need for insulin increases and its effectiveness decreases.
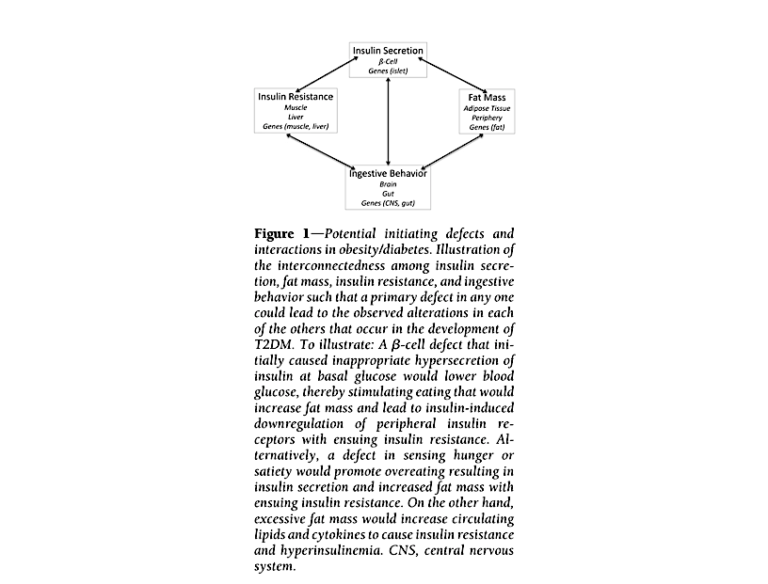
The clinical manifestations of diabetes may be explained by an opposite causal pathway. Elevated insulin levels have been shown to cause insulin resistance in the liver, peripheral tissues (i.e., fat and muscle), and brain (3); the latter has been linked to ineffective satiety signals, which leads to overeating. Mouse and human studies (4) have shown that dosing healthy subjects with insulin leads to development of insulin resistance and obesity. If hyperinsulinemia (i.e., high insulin levels) are the primary cause of diabetes, insulin resistance is a downstream consequence, with muscle and fat cells becoming insulin resistant to avoid taking up too much glucose from the blood and causing hypoglycemia. Figure 1 below, from Corkey’s paper, summarizes the ways a primary defect in insulin secretion could lead to the other clinical observations commonly associated with diabetes.
Additional evidence suggests the most consistent difference between diabetic and healthy subjects is elevated insulin levels — more so than elevated glucose levels. Fasting insulin levels in subjects with high blood sugar (fasting glucose > 140 mg/dL) are nine times higher than in healthy subjects (5). Additionally, insulin elevates more dramatically and more quickly in obese, pre-diabetic and diabetic subjects (progressively between those groups) than in healthy subjects, with maximum insulin concentrations twice as high in diabetic subjects as healthy subjects (6). The glucose and insulin behavior of diabetic subjects is summarized in the figures directly below.
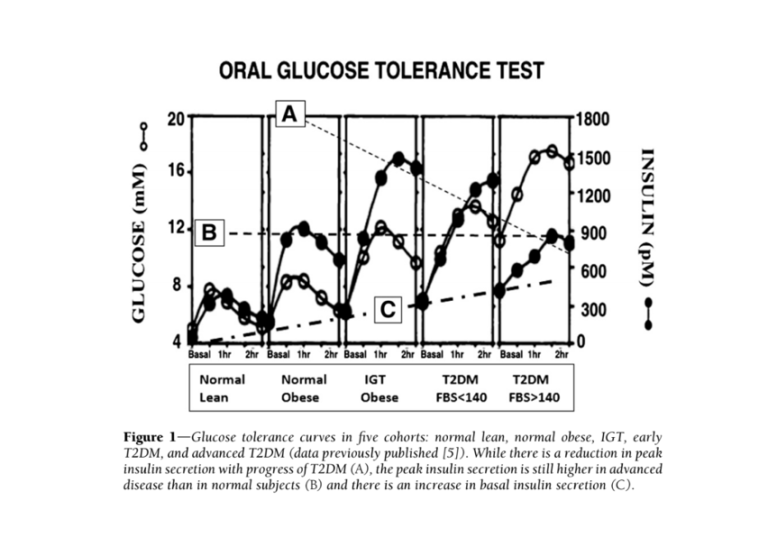
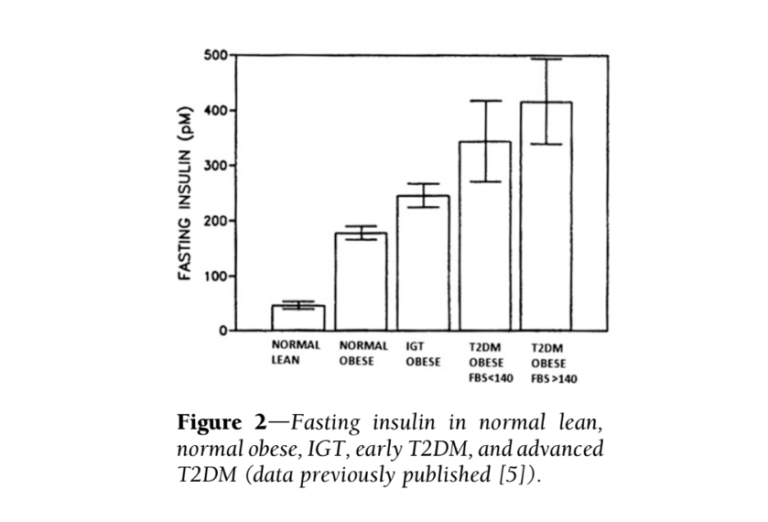
Taken together, this evidence suggests high insulin levels directly contribute to the development and/or progression of diabetes. If this is true, it suggests elements of the standard-of-care treatment, particularly the use of injected insulin to achieve normal blood glucose levels, are counterproductive and in fact contribute to diabetes progression.
As Pories notes, “We do not treat hyperthyroidism with thyroxine or Cushing syndrome with cortisone; should we be treating T2DM, a disease characterized by hyperinsulinemia, with insulin?” (2442).
Corkey argues this initial increase in insulin secretion may be the result of factors introduced to our diet and environment since the 1960s, such as pesticides and plastics. These factors, she argues, cause oxidative damage to pancreatic beta cells and lead to hypersecretion of insulin even when blood glucose levels are normal (7). Pories instead argues hyperinsulinemia results from defective signaling between the gut and pancreas, as evidenced by the rapid resolution of hyperinsulinemia and other clinical symptoms of diabetes after roux-en-y gastric bypass surgery, which rearranges the gut in ways that directly affect relevant signaling pathways (8).
While these two specific explanations are preliminary, the broader hypothesis that diabetes is a disease driven by both hyperinsulinemia and hyperglycemia — and that interventions that increase insulin to control glucose therefore may be counterproductive — is well substantiated. This hypothesis would suggest interventions that specifically reduce blood insulin levels, such as dietary carbohydrate restriction, may be particularly effective in preventing the progression of or even reversing diabetes.
Notes
- Prevalence of high body mass index in US children and adolescents, 2007-2008
- Prevention and treatment of the complications of diabetes mellitus
- Insulin-dependent regulation of insulin receptor concentrations: A direct demonstration in cell culture; Central insulin administration reduces neuropeptide Y mRNA expression in the acrcuate nucleus of food-deprived lean but not obese Zucker rats; Intraventricular insnulin and the level of maintained body weight in rats
- Effect of chronic insulin administration on food intake and body weight in rats; Antiobesity effect of diazoxide in obese Zucker rats; Beneficial effect of diazoxide in obese hyperinsulinemic adults; Diazoxide down-regulates leptin and lipid metabolizing enzymes in adipose tissue of Zucker rats; Concomitannt food intake and adipose tussue responses under chronic insulin infusion in rats; Effect of sustained physiologic hyperinnsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man
- Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up
- This begins to reverse in late-stage diabetes in which gradual pancreatic damage impairs insulin production and release; at this point, Type 2 diabetes begins to resemble Type 1 diabetes.
- Reactive oxygen species stimulate insulin secretion in rat pancreatic islets: Studies using monoleoyl-glycerol; Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways, etc.
- Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes; Bariatric surgery versus intensive medical therapy in obese patients with diabetes; Roux-en-Y gastric bypass corrects hyperinsulinemia: Implications for the remission of type 2 diabetes; Mechanism for improved insulin sensitivity after gastric bypass surgery, etc.
Comments on Diabetes: Have We Got It All Wrong?
Good article, but the following sentence prompted some questions. "Since the 1960s, the prevalence of diabetes in the U.S. population has increased from ~5% to more than 15% (1)." I can’t find any diabetes prevalence rates in the cited reference.
Also, the “more than 15%” statistic doesn’t seem to me to reconcile with other published statistics. I think the CDC’s most recent version of its National Diabetes Statistics Report is for 2017 (https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf). Unfortunately it uses 2015 data. From the report:
“Prevalence of Both Diagnosed and Undiagnosed Diabetes
- An estimated 30.3 million people of all ages—or 9.4% of the U.S. population—had diabetes in 2015.
- This total included 30.2 million adults aged 18 years or older (12.2% of all U.S. adults), of which 7.2 million (23.8%) were not aware of or did not report having diabetes.”
To estimate an update of the 2015 "9.4%":
In 2019, there are 31.0 million U.S. adults (aged 20-79) with diabetes (per the International Diabetes Federation Diabetes Atlas, 9th edition 2019, https://diabetesatlas.org/en/sections/demographic-and-geographic-outline.html). Even if we assume that 33% of the 12.7 million U.S. adults currently over age 79 have diabetes, and assume that 0.2 million children and adolescents do, we still only get a total of 35.5 million with diabetes in 2019. Divided by a U.S. population of 329 million, that gives a (probably high) estimate of 10.7%.
???
Downstream isn’t upstream.
This analogy by Dr Fung is easy to understand.
https://thefastingmethod.com/insulin-resistance-good-t2d-7/
Diabetes: Have We Got It All Wrong?
2