Question: What are some of the morphological changes associated with the diabetic lung?
Takeaway: This 1997 study in hamsters found multiple changes in the cells and structures of the diabetic lung, including increased macrophage adherence to the alveolar lumen, thickening of the alveolar endothelium, and enlargement of the extracellular matrix. These changes may explain the impairments in pulmonary function seen in diabetics.
Diabetes is associated with a variety of pulmonary defects including reduced expiratory volume (1), increased venous resistance (2) and changes to IGF-1 and ACE levels in the lungs (3). Diabetics are more susceptible to pulmonary infections (4). This 1997 hamster study sought to better understand the specific morphological changes of the lung associated with diabetes.
Hamsters were divided into three groups: those given streptozotocin (which induces diabetes), those given streptozotocin plus a high-cholesterol diet, and controls. Note that in hamsters, unlike in humans, excess cholesterol consumption induces a dramatic elevation in blood cholesterol levels. The hamsters were followed for 24 weeks.
After only six weeks of hyperglycemia, morphological changes were observed in the hamsters’ lung cells and microstructures. These changes included:
- Increased numbers of biosynthetic organelles (endoplasmic reticulum, Golgi apparatus)
- Reduced numbers of organelles involved in degradation (lysosomes)
- Increased adherence of macrophages to the alveolar lumen
- Thickening of the basal lamina between cells within the alveoli
- Increased extracellular matrix in the cellular interstitium (i.e., the area between cells)
- An uneven distribution of anionic charge throughout the cellular membranes of alveolar cells
These changes were associated with narrowing or collapse of 35% of capillaries and 30% of alveoli by the 18th week. Many of these changes are similar to those previously seen in the arterial, myocardial, and retinal endothelium of diabetics (5). Similar changes were seen in the hamsters that were both diabetic and hyperlipidemic, with the speed and degree of progression generally greater in the hyperlipidemic hamsters.
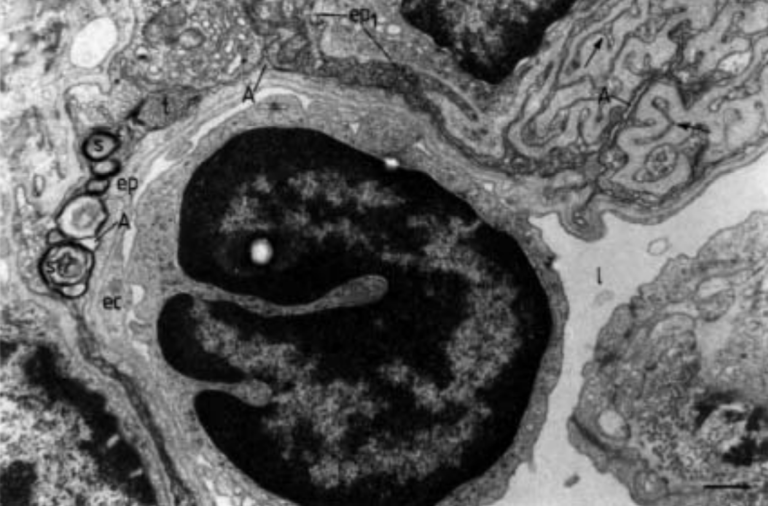
Fig. 5: Compressed lung alveolar space of a 24-week-old diabetic hamster. Over a large surface, the alveolar space (A) becomes collapsed, displaying compressed surfactant, which appears as tubular myelin (t) or as a multilayered structure (s). Note the osmiophilic type I epithelial cell (ep1), whose contorted contour (arrows) is accompanied by interstitial tissue (is), as well as the presence of apparently frequent collagen bundles (arrowheads), and elastin (e). l: vascular lumen; ec: endothelial cell; ep: type I epithelial cell. (Internal scale bar=0.430 µm).
Changes to the thickness and structure of the intracellular and extracellular matrices would be expected to impair alveolar gas transport. The uneven distribution of anionic charge would be expected to impair the adherence of circulating blood cells with the capillary lumen. Taken together, the authors conclude these changes contribute to impaired pulmonary function in the diabetic lung and indicate a direct link between diabetes and loss of lung function.
Notes
- Indications of reduced pulmonary function in type I (insulin-dependent) diabetes mellitus
- Alteration of the segmental vascular resistance in the pulmonary circulation of diabetic rats
- Diminished levels of insulin-like growth factor-I in lungs in streptozotocin-induced diabetes: Relation to nutritional status and growth; Angiotensin-converting enzyme activity in the serum, lung and kidney of diabetic rats
- Impaired immune responses in diabetes mellitus: Analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections; Diabetic ketoacidosis associated with pulmonary coccidioidomycosis
- Pathobiochemistry of combined diabetes and atherosclerosis studied on a novel animal model: The hyperlipidemic-hyperglycemic hamster; Reduced negative surface charge on arterial endothelium of diabetic rats; The pathomorphological alterations of endocardial endothelium in experimental diabetes, and diabetes associated with hyperlipidemia
Comments on Alterations of Lung Structure in Experimental Diabetes
And, in light of the Kohio et al study (cited below) showing that elevated tissue glucose increases the ability of influenza viruses to adhere and penetrate the endothelial cells, the possibility is raised that in the current pandemic it may be easier for SARS-CoV2 to infect the lungs of diabetics in the first place and once accomplished, with all those macrophages poised there to attack, a higher chance of overwhelming inflammatory response throughout the alveoli and the greater risk of the respiratory distress that leads to the ventilator. Certainly that's what we've seen occurring re metabolic syndrome and obesity (itself part of metabolic syndrome) driving up the risk of bad covid-19 outcomes in even those younger than 'elderly'.
(Kohio, H.P., Adamson, A.L., Glycolytic control of vacuolar-type ATPase activity: A mechanism to regulate
influenza viral infection. Virology (2013), http://dx.doi.org/10.1016/j.virol.2013.06.026i)
Exactly. There are two (maybe more, but two I can think of) axes which could explain the clearly observed phenomenon that diabetics have a higher rate of COVID morbidity and mortality than nondiabetics:
- Given the same exposure, diabetics are more likely to be infected;
- Given the same rate of infection, diabetics are more likely to be harmed
I'm slowly working my way through the data on #2, but yes, this paper and others like it suggest specific morphological changes in diabetics that could explain #1. If both were true, that would position diabetes as an extremely harmful condition, particularly if large-scale infections like COVID and like the last few flu seasons we have experienced increasingly become the norm. The questions that follow then, for me, are:
- What aspect of diabetes causes this? Hyperglycemia? Hyperinsulinemia? Chronic and/or acute? Other moderating factors such as inflammation, oxidation, etc? Lipid changes? And, based on this, how broad is this effect? (Everybody who is insulin resistant? Everybody who is hyperglycemic?)
- How quickly can it be reversed? We know diabetes can be reversed through aggressive action in a matter of weeks, except in those who have been diabetic for many years. Do these risk factors reverse with it?
- How risky are these conditions when the increased risk attributable to various metabolic defects are removed? i.e., to what extent is the high morbidity and mortality we have seen from many recent flu seasons the result of increased population vulnerability, rather than due to the infectious agents themselves?
The answer to these questions should color how we think of chronic disease, and how we ought to respond to the chronic disease epidemics at an individual and a societal level in light of the infectious disease epidemics we are now seeing.
Alterations of Lung Structure in Experimental Diabetes
2